We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
3ve1
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
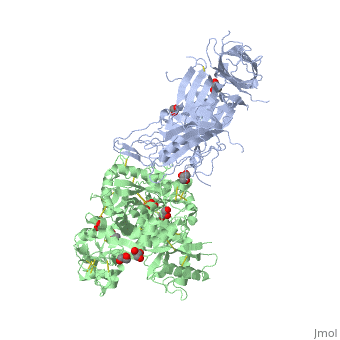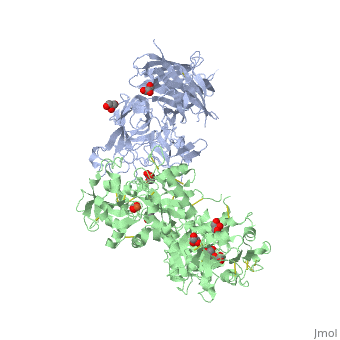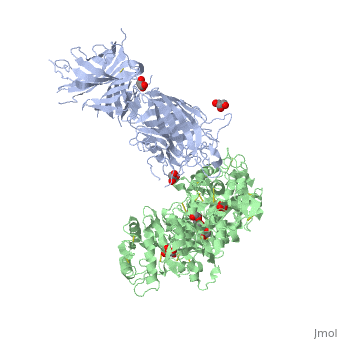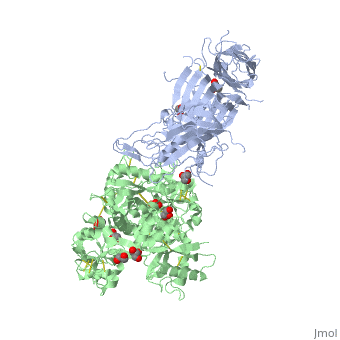
| - | + | ==The 2.9 angstrom crystal structure of Transferrin binding protein B (TbpB) from serogroup B M982 Neisseria meningitidis in complex with human transferrin== | |
| - | + | <StructureSection load='3ve1' size='340' side='right' caption='[[3ve1]], [[Resolution|resolution]] 2.96Å' scene=''> | |
| - | + | == Structural highlights == | |
| - | + | <table><tr><td colspan='2'>[[3ve1]] is a 4 chain structure with sequence from [http://en.wikipedia.org/wiki/Homo_sapiens Homo sapiens] and [http://en.wikipedia.org/wiki/Neisseria_meningitidis_serogroup_b Neisseria meningitidis serogroup b]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=3VE1 OCA]. For a <b>guided tour on the structure components</b> use [http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=3VE1 FirstGlance]. <br> | |
| - | ==Disease== | + | </td></tr><tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat"><scene name='pdbligand=CO3:CARBONATE+ION'>CO3</scene>, <scene name='pdbligand=FE:FE+(III)+ION'>FE</scene>, <scene name='pdbligand=GOL:GLYCEROL'>GOL</scene></td></tr> |
| + | <tr id='related'><td class="sblockLbl"><b>[[Related_structure|Related:]]</b></td><td class="sblockDat">[[3ve2|3ve2]]</td></tr> | ||
| + | <tr id='gene'><td class="sblockLbl"><b>[[Gene|Gene:]]</b></td><td class="sblockDat">tbpB, tbp2 ([http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&srchmode=5&id=491 Neisseria meningitidis serogroup B]), TF, PRO1400 ([http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&srchmode=5&id=9606 Homo sapiens])</td></tr> | ||
| + | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=3ve1 FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=3ve1 OCA], [http://www.rcsb.org/pdb/explore.do?structureId=3ve1 RCSB], [http://www.ebi.ac.uk/pdbsum/3ve1 PDBsum]</span></td></tr> | ||
| + | </table> | ||
| + | == Disease == | ||
[[http://www.uniprot.org/uniprot/TRFE_HUMAN TRFE_HUMAN]] Defects in TF are the cause of atransferrinemia (ATRAF) [MIM:[http://omim.org/entry/209300 209300]]. Atransferrinemia is rare autosomal recessive disorder characterized by iron overload and hypochromic anemia.<ref>PMID:11110675</ref> <ref>PMID:15466165</ref> | [[http://www.uniprot.org/uniprot/TRFE_HUMAN TRFE_HUMAN]] Defects in TF are the cause of atransferrinemia (ATRAF) [MIM:[http://omim.org/entry/209300 209300]]. Atransferrinemia is rare autosomal recessive disorder characterized by iron overload and hypochromic anemia.<ref>PMID:11110675</ref> <ref>PMID:15466165</ref> | ||
| - | + | == Function == | |
| - | ==Function== | + | |
[[http://www.uniprot.org/uniprot/TRFE_HUMAN TRFE_HUMAN]] Transferrins are iron binding transport proteins which can bind two Fe(3+) ions in association with the binding of an anion, usually bicarbonate. It is responsible for the transport of iron from sites of absorption and heme degradation to those of storage and utilization. Serum transferrin may also have a further role in stimulating cell proliferation. | [[http://www.uniprot.org/uniprot/TRFE_HUMAN TRFE_HUMAN]] Transferrins are iron binding transport proteins which can bind two Fe(3+) ions in association with the binding of an anion, usually bicarbonate. It is responsible for the transport of iron from sites of absorption and heme degradation to those of storage and utilization. Serum transferrin may also have a further role in stimulating cell proliferation. | ||
| + | <div style="background-color:#fffaf0;"> | ||
| + | == Publication Abstract from PubMed == | ||
| + | Neisseria meningitidis, the causative agent of bacterial meningitis, acquires the essential element iron from the host glycoprotein transferrin during infection through a surface transferrin receptor system composed of proteins TbpA and TbpB. Here we present the crystal structures of TbpB from N. meningitidis in its apo form and in complex with human transferrin. The structure reveals how TbpB sequesters and initiates iron release from human transferrin. | ||
| - | + | The structural basis of transferrin sequestration by transferrin-binding protein B.,Calmettes C, Alcantara J, Yu RH, Schryvers AB, Moraes TF Nat Struct Mol Biol. 2012 Feb 19;19(3):358-60. doi: 10.1038/nsmb.2251. PMID:22343719<ref>PMID:22343719</ref> | |
| - | + | ||
| - | == | + | From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> |
| - | + | </div> | |
| + | == References == | ||
| + | <references/> | ||
| + | __TOC__ | ||
| + | </StructureSection> | ||
[[Category: Homo sapiens]] | [[Category: Homo sapiens]] | ||
[[Category: Neisseria meningitidis serogroup b]] | [[Category: Neisseria meningitidis serogroup b]] | ||
| - | [[Category: Calmettes, C | + | [[Category: Calmettes, C]] |
| - | [[Category: Moraes, T F | + | [[Category: Moraes, T F]] |
[[Category: Host pathogen interaction]] | [[Category: Host pathogen interaction]] | ||
[[Category: Iron acquisition]] | [[Category: Iron acquisition]] | ||
Revision as of 06:30, 21 December 2014
The 2.9 angstrom crystal structure of Transferrin binding protein B (TbpB) from serogroup B M982 Neisseria meningitidis in complex with human transferrin
| |||||||||||