We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 967
From Proteopedia
(Difference between revisions)
| Line 31: | Line 31: | ||
=== Several interactions between the subunits === | === Several interactions between the subunits === | ||
H2C protein is found in the middle of the elongated complex structure, flanked by H2A and H2B proteins on the ends. | H2C protein is found in the middle of the elongated complex structure, flanked by H2A and H2B proteins on the ends. | ||
| - | The complex is stabilized by the intimately interwoven architecture of H2B and H2C: The N-terminal region of H2B protein (amino acids 1-92) weaves together with H2C domain to form 3 β-barrels, called <scene name='60/604486/Triple_barrel/2'>“triple barrel”</scene><ref name ="ref9"> Nicholson, Allen W. Ribonucleases. Springer Science & Business Media, 2011.</ref>. This triple barrel is formed from a total of <scene name='60/604486/Triple_barrel/1'>18 β-sheets</scene> and produces a pseudo-2-fold axis of symmetry along the central barrel. Also, it permits to leave the mostly α-helical C-terminal region of H2B available for potential interactions with other protein (for example the PCNA protein). Finally, it has been found that the motif provides a platform for securely binding the H2A protein: the side and end of the first barrel in the subcomplex H2B/H2C form a <scene name='60/604486/Tight_interface_h2ah2c/2'>tight interface</scene> with amino acids 197-258 in the C-terminal region of H2A protein. This interface is mainly composed of hydrophobic residues | + | The complex is stabilized by the intimately interwoven architecture of H2B and H2C: The N-terminal region of H2B protein (amino acids 1-92) weaves together with H2C domain to form 3 β-barrels, called <scene name='60/604486/Triple_barrel/2'>“triple barrel”</scene><ref name ="ref9"> Nicholson, Allen W. Ribonucleases. Springer Science & Business Media, 2011.</ref>. This triple barrel is formed from a total of <scene name='60/604486/Triple_barrel/1'>18 β-sheets</scene> and produces a pseudo-2-fold axis of symmetry along the central barrel. Also, it permits to leave the mostly α-helical C-terminal region of H2B available for potential interactions with other protein (for example the PCNA protein). Finally, it has been found that the motif provides a platform for securely binding the H2A protein: the side and end of the first barrel in the subcomplex H2B/H2C form a <scene name='60/604486/Tight_interface_h2ah2c/2'>tight interface </scene> with amino acids 197-258 in the C-terminal region of H2A protein. This interface is mainly composed of hydrophobic residues |
| Line 37: | Line 37: | ||
It has been proved that the position of RNA/DNA complex in the active site cleft is determined by several favorable electrostatic interactions between the nucleic acid and positively charged amino acids of the protein<ref name = "ref2" />. | It has been proved that the position of RNA/DNA complex in the active site cleft is determined by several favorable electrostatic interactions between the nucleic acid and positively charged amino acids of the protein<ref name = "ref2" />. | ||
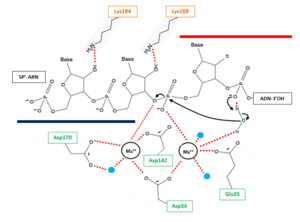
| - | The β6-α6 loop of the H2A protein could play a role in substrate recognition: the minor groove of the double helix molecule straddles this area of the protein, which results in a non-sequence specific cleavage by the enzyme. Moreover, the β6-α6 loop contains a<scene name='60/604486/Site_actif_dna_recongnition/1'>Lysine amino acid in position 128</scene>, which might act as a sensor for the hybrid by forming an interaction with the 2’-hydroxyl group of the ribose in the 3’ nucleotide of the RNA primer in the RNA-DNA hybrid ('''Figure 2'''). Therefore, since DNA does not contain a 2’-hydroxyl group in it nucleotide sequence, the RNase H2 can only recognize RNA in the hybrid: only ribonucleotides of the RNA strand are positioned in the active site. The RNA-DNA hybrid is placed such that the target phosphodiester bond between the RNA and DNA parts of the hybrid is in the proper orientation for nucleophile attack by a two-metal ion mechanism. | + | The β6-α6 loop of the H2A protein could play a role in substrate recognition: the minor groove of the double helix molecule straddles this area of the protein, which results in a non-sequence specific cleavage by the enzyme. Moreover, the β6-α6 loop contains a <scene name='60/604486/Site_actif_dna_recongnition/1'>Lysine amino acid in position 128</scene>, which might act as a sensor for the hybrid by forming an interaction with the 2’-hydroxyl group of the ribose in the 3’ nucleotide of the RNA primer in the RNA-DNA hybrid .Another Lysine might be involved in the recognition of nucleic acids, indeed <scene name='60/604486/Site_actif_dna_recongnition/1'>Lysine 184</scene> might be able to recognize the 2'-hydroxyl groupe of the ribose of the amino acid at 5'end of the RNA ('''Figure 2'''). Therefore, since DNA does not contain a 2’-hydroxyl group in it nucleotide sequence, the RNase H2 can only recognize RNA in the hybrid: only ribonucleotides of the RNA strand are positioned in the active site. The RNA-DNA hybrid is placed such that the target phosphodiester bond between the RNA and DNA parts of the hybrid is in the proper orientation for nucleophile attack by a two-metal ion mechanism. |
It is important to notice that the Mammalian RNase H2 contains only one cleft with the active site for substrate binding: RNase H2 may recognize single ribonucleotide within a DNA duplex that have a B-form helical structure, as well as longer RNA in RNA-DNA hybrid which adopts intermediate A/B form structure. Thus, the RNase H2 enzyme needs to bind both conformations to able to fully complete all its roles. | It is important to notice that the Mammalian RNase H2 contains only one cleft with the active site for substrate binding: RNase H2 may recognize single ribonucleotide within a DNA duplex that have a B-form helical structure, as well as longer RNA in RNA-DNA hybrid which adopts intermediate A/B form structure. Thus, the RNase H2 enzyme needs to bind both conformations to able to fully complete all its roles. | ||
| Line 49: | Line 49: | ||
The hydrolysis can be decomposed in 3 steps: | The hydrolysis can be decomposed in 3 steps: | ||
* '''1''' : Deprotonation of a water molecule coordinated to the metal MB++ to form a nucleophile OH- ion. This hydroxide ion will then be properly oriented for an in-line nucleophilic attack of the target phosphate. The deprotonation mechanism has not been elucidated yet but two hypothesis can explain this step. According the first one, the metal MB++ might be responsible for the generation of water nucleophile. The other one involve a participation of the pro-R oxygen of the phosphate immediately to the 3’ side of the scissile bond, which is likely to serve as a general base for deprotonation (with transfer of the H+ to the solvent).This pro-R oxygen is also thought to play a role in the proper orientation of the hydroxide ion. | * '''1''' : Deprotonation of a water molecule coordinated to the metal MB++ to form a nucleophile OH- ion. This hydroxide ion will then be properly oriented for an in-line nucleophilic attack of the target phosphate. The deprotonation mechanism has not been elucidated yet but two hypothesis can explain this step. According the first one, the metal MB++ might be responsible for the generation of water nucleophile. The other one involve a participation of the pro-R oxygen of the phosphate immediately to the 3’ side of the scissile bond, which is likely to serve as a general base for deprotonation (with transfer of the H+ to the solvent).This pro-R oxygen is also thought to play a role in the proper orientation of the hydroxide ion. | ||
| - | * '''2''' : In line attack by hydroxide ion of the target phosphate. During this step, a pentacovalent phosphate (transition state) is formed and stabilized by metal MA++ | + | * '''2''' : In line attack by hydroxide ion of the target phosphate. During this step, a pentacovalent phosphate (transition state) is formed and stabilized by metal MA++. The metal MA++ interacts with both the nonbridging and 3' bridging oxygen (figure ###) and the strain induced by this double briding is thought to accelerate the hydrolysis rate. This step release a 3’ oxyanion stabilized by metal MA++ acting as a Lewis acid. ['''Rajouter figure'''] |
* '''3''' : The cleaved phosphate cannot simultaneously coordinate the two metal ions anymore, and likely one of the metal ions leave the active site which triggers a release of cleave product. | * '''3''' : The cleaved phosphate cannot simultaneously coordinate the two metal ions anymore, and likely one of the metal ions leave the active site which triggers a release of cleave product. | ||
| - | This is a sample scene created with SAT to <scene name="/12/3456/Sample/1">color</scene> by Group, and another to make <scene name="/12/3456/Sample/2">a transparent representation</scene> of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes. | ||
</StructureSection> | </StructureSection> | ||
Revision as of 09:46, 10 January 2015
| This Sandbox is Reserved from 15/11/2014, through 15/05/2015 for use in the course "Biomolecule" taught by Bruno Kieffer at the Strasbourg University. This reservation includes Sandbox Reserved 951 through Sandbox Reserved 975. |
To get started:
More help: Help:Editing |
Structure of the Mouse RNase H2 Complex
| |||||||||||
References
- ↑ http://genome-euro.ucsc.edu/cgi-bin/hgTracks?clade=mammal&org=Mouse&db=mm10&position=RnaseH2&hgt.positionInput=RnaseH2&hgt.suggestTrack=knownGene&Submit=submit&hgsid=201143152_yP1Xd4bMnHS7DV0d3VcqpDSxzzuQ&pix=1563
- ↑ 2.0 2.1 Rychlik, Monika P., Hyongi Chon, Susana M. Cerritelli, Paulina Klimek, Robert J. Crouch, and Marcin Nowotny. “Crystal Structures of RNase H2 in Complex with Nucleic Acid Reveal the Mechanism of RNA-DNA Junction Recognition and Cleavage.” Molecular Cell 40, no. 4 (November 24, 2010): 658–70. doi:10.1016/j.molcel.2010.11.001.
- ↑ Sparks, Justin L., Hyongi Chon, Susana M. Cerritelli, Thomas A. Kunkel, Erik Johansson, Robert J. Crouch, and Peter M. Burgers. “RNase H2-Initiated Ribonucleotide Excision Repair.” Molecular Cell 47, no. 6 (September 28, 2012): 980–86. doi:10.1016/j.molcel.2012.06.035.
- ↑ 4.0 4.1 4.2 Bubeck, Doryen, Martin A. M. Reijns, Stephen C. Graham, Katy R. Astell, E. Yvonne Jones, and Andrew P. Jackson. “PCNA Directs Type 2 RNase H Activity on DNA Replication and Repair Substrates.” Nucleic Acids Research 39, no. 9 (May 2011): 3652–66. doi:10.1093/nar/gkq980.
- ↑ Shaban, Nadine M., Scott Harvey, Fred W. Perrino, and Thomas Hollis. “The Structure of the Mammalian RNase H2 Complex Provides Insight into RNA•DNA Hybrid Processing to Prevent Immune Dysfunction.” Journal of Biological Chemistry 285, no. 6 (February 5, 2010): 3617–24. doi:10.1074/jbc.M109.059048.
- ↑ http://www.uniprot.org/uniprot/Q9CWY8
- ↑ http://www.uniprot.org/uniprot/Q80ZV0
- ↑ http://www.uniprot.org/uniprot/Q9CQ18
- ↑ Nicholson, Allen W. Ribonucleases. Springer Science & Business Media, 2011.
Proteopedia page contributors and editors
Anaïs Bourbigot & Valériane Keïta