Factor IX
From Proteopedia
(Difference between revisions)
| Line 46: | Line 46: | ||
[[Image:Research_02_big4.JPG|frame|right|http://www.molecular-haemostasis.de/grfx/pix/]] | [[Image:Research_02_big4.JPG|frame|right|http://www.molecular-haemostasis.de/grfx/pix/]] | ||
| + | {{Clear}} | ||
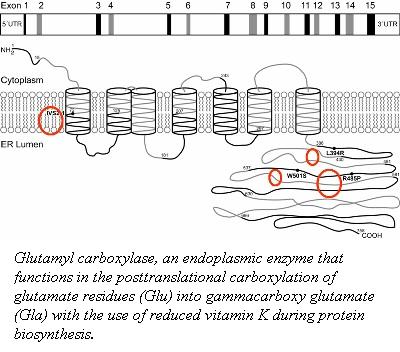
The post-translational modification of the glutamic acid residues of the FIX polypeptide is carried out by vitamin K-dependent ''γ''-glutamyl carboxylase <ref>PMID:902786</ref>. Vitamin K-dependent carboxylase is a membrane associated protein in the endoplasmic reticulum. It converts a multiple of glutamic acid residues which are located within 40 residues of a propeptide-containing sequence into ''γ''-carboxyglutamate <ref>PMID:8530480</ref>. | The post-translational modification of the glutamic acid residues of the FIX polypeptide is carried out by vitamin K-dependent ''γ''-glutamyl carboxylase <ref>PMID:902786</ref>. Vitamin K-dependent carboxylase is a membrane associated protein in the endoplasmic reticulum. It converts a multiple of glutamic acid residues which are located within 40 residues of a propeptide-containing sequence into ''γ''-carboxyglutamate <ref>PMID:8530480</ref>. | ||
| Line 103: | Line 104: | ||
-------------- | -------------- | ||
http://www.natuurlijkerwijs.com/english/ ]] | http://www.natuurlijkerwijs.com/english/ ]] | ||
| - | + | {{Clear}} | |
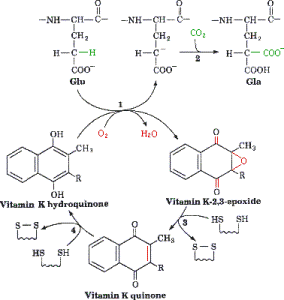
<span style="color:Brown">'''''γ''-Carboxylation Reaction'''</span> is catalyzed by the γ-glutamyl carboxylase in the ER and requires a reduced form of vitamin K, oxygen, and carbon dioxide<ref>PMID:10068650</ref>. The reaction is initiated by the removal of a hydrogen atom at the γ position of glutamate. This reaction creates a carbon ion that reacts with carbon dioxide thus forming γ-carboxy glutamic acid. As the protein is carboxylated using carbon dioxide the reduced vitamin K (hydroquinone) is oxidized to epoxide. | <span style="color:Brown">'''''γ''-Carboxylation Reaction'''</span> is catalyzed by the γ-glutamyl carboxylase in the ER and requires a reduced form of vitamin K, oxygen, and carbon dioxide<ref>PMID:10068650</ref>. The reaction is initiated by the removal of a hydrogen atom at the γ position of glutamate. This reaction creates a carbon ion that reacts with carbon dioxide thus forming γ-carboxy glutamic acid. As the protein is carboxylated using carbon dioxide the reduced vitamin K (hydroquinone) is oxidized to epoxide. | ||
| Line 150: | Line 151: | ||
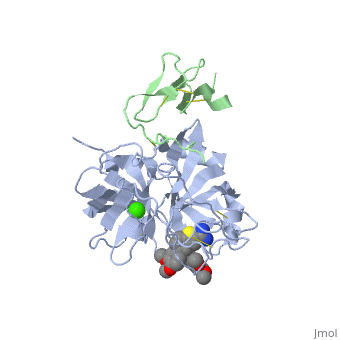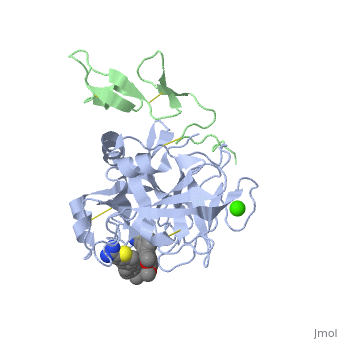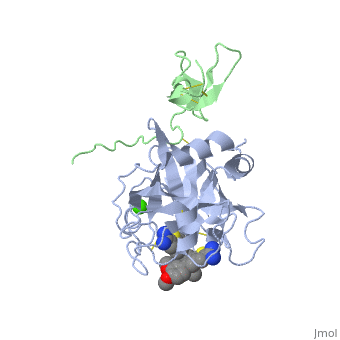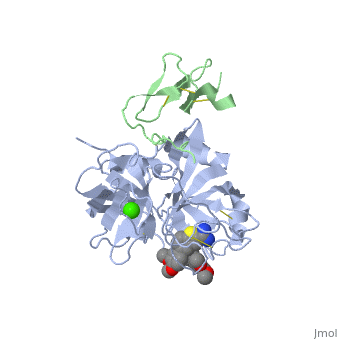
The catalytic domain of FIX is composed of two β-<scene name='Factor_IX/Rfnscene/3'>barrel</scene> subdomains that form an active site at their interface. The active site is located at the junction of these β-barrels. The EGF-2 domain is connected to the catalytic domain through a disulfide bridge and is opposite to the active site. There are three disulfide bonds, and the C-terminus contains helical structures that run across the N-terminus of the β-barrel. The catalytic domain contains a calcium binding site that exposes the <scene name='Factor_IX/Rfnscene_5/1'>148-loop</scene> for proteolytic cleavage. This calcium ion is stabilized by Glu-70, Glu-77, Glu-80 and a main chain oxygens of Asn-72 and Glu-75. This site seems to be preformed, unlike the calcium binding sites that are generated in the Gla domain upon calcium binding. | The catalytic domain of FIX is composed of two β-<scene name='Factor_IX/Rfnscene/3'>barrel</scene> subdomains that form an active site at their interface. The active site is located at the junction of these β-barrels. The EGF-2 domain is connected to the catalytic domain through a disulfide bridge and is opposite to the active site. There are three disulfide bonds, and the C-terminus contains helical structures that run across the N-terminus of the β-barrel. The catalytic domain contains a calcium binding site that exposes the <scene name='Factor_IX/Rfnscene_5/1'>148-loop</scene> for proteolytic cleavage. This calcium ion is stabilized by Glu-70, Glu-77, Glu-80 and a main chain oxygens of Asn-72 and Glu-75. This site seems to be preformed, unlike the calcium binding sites that are generated in the Gla domain upon calcium binding. | ||
| - | + | </StructureSection> | |
==3D structures of factor IX== | ==3D structures of factor IX== | ||
Revision as of 13:46, 22 January 2015
| |||||||||||
3D structures of factor IX
Updated on 22-January-2015
Additional Resources
For additional information, see: Colored & Bioluminescent Proteins
For additional information, see: Hemophilia
References
- ↑ Spitzer SG, Kuppuswamy MN, Saini R, Kasper CK, Birktoft JJ, Bajaj SP. Factor IXHollywood: substitution of Pro55 by Ala in the first epidermal growth factor-like domain. Blood. 1990 Oct 15;76(8):1530-7. PMID:2169923
- ↑ Schmidt AE, Bajaj SP. Structure-function relationships in factor IX and factor IXa. Trends Cardiovasc Med. 2003 Jan;13(1):39-45. PMID:12554099
- ↑ Zhong D, Bajaj MS, Schmidt AE, Bajaj SP. The N-terminal epidermal growth factor-like domain in factor IX and factor X represents an important recognition motif for binding to tissue factor. J Biol Chem. 2002 Feb 1;277(5):3622-31. Epub 2001 Nov 26. PMID:11723140 doi:10.1074/jbc.M111202200
- ↑ Perona JJ, Craik CS. Evolutionary divergence of substrate specificity within the chymotrypsin-like serine protease fold. J Biol Chem. 1997 Nov 28;272(48):29987-90. PMID:9374470
- ↑ Ludwig M, Sabharwal AK, Brackmann HH, Olek K, Smith KJ, Birktoft JJ, Bajaj SP. Hemophilia B caused by five different nondeletion mutations in the protease domain of factor IX. Blood. 1992 Mar 1;79(5):1225-32. PMID:1346975
- ↑ Murav'ev IA, Mar'iasis ED, Starokozhko LE, Chebotarev VV, Krasova TG. [Determination of the biologic availability of preparations for topical use by experimental dermatologic methods] Farmatsiia. 1977 Jul-Aug;26(4):15-9. PMID:902786
- ↑ Morris DP, Stevens RD, Wright DJ, Stafford DW. Processive post-translational modification. Vitamin K-dependent carboxylation of a peptide substrate. J Biol Chem. 1995 Dec 22;270(51):30491-8. PMID:8530480
- ↑ Pan LC, Price PA. The propeptide of rat bone gamma-carboxyglutamic acid protein shares homology with other vitamin K-dependent protein precursors. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6109-13. PMID:3875856
- ↑ Knobloch JE, Suttie JW. Vitamin K-dependent carboxylase. Control of enzyme activity by the "propeptide" region of factor X. J Biol Chem. 1987 Nov 15;262(32):15334-7. PMID:2890628
- ↑ Knobloch JE, Suttie JW. Vitamin K-dependent carboxylase. Control of enzyme activity by the "propeptide" region of factor X. J Biol Chem. 1987 Nov 15;262(32):15334-7. PMID:2890628
- ↑ Cheung A, Engelke JA, Sanders C, Suttie JW. Vitamin K-dependent carboxylase: influence of the "propeptide" region on enzyme activity. Arch Biochem Biophys. 1989 Nov 1;274(2):574-81. PMID:2802629
- ↑ Lin PJ, Jin DY, Tie JK, Presnell SR, Straight DL, Stafford DW. The putative vitamin K-dependent gamma-glutamyl carboxylase internal propeptide appears to be the propeptide binding site. J Biol Chem. 2002 Aug 9;277(32):28584-91. Epub 2002 May 28. PMID:12034728 doi:10.1074/jbc.M202292200
- ↑ Sanford DG, Kanagy C, Sudmeier JL, Furie BC, Furie B, Bachovchin WW. Structure of the propeptide of prothrombin containing the gamma-carboxylation recognition site determined by two-dimensional NMR spectroscopy. Biochemistry. 1991 Oct 15;30(41):9835-41. PMID:1911775
- ↑ Lin PJ, Jin DY, Tie JK, Presnell SR, Straight DL, Stafford DW. The putative vitamin K-dependent gamma-glutamyl carboxylase internal propeptide appears to be the propeptide binding site. J Biol Chem. 2002 Aug 9;277(32):28584-91. Epub 2002 May 28. PMID:12034728 doi:10.1074/jbc.M202292200
- ↑ Furie B, Bouchard BA, Furie BC. Vitamin K-dependent biosynthesis of gamma-carboxyglutamic acid. Blood. 1999 Mar 15;93(6):1798-808. PMID:10068650
- ↑ Thompson AR. Structure, function, and molecular defects of factor IX. Blood. 1986 Mar;67(3):565-72. PMID:3511981
- ↑ Stenflo J, Fernlund P, Egan W, Roepstorff P. Vitamin K dependent modifications of glutamic acid residues in prothrombin. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2730-3. PMID:4528109
- ↑ Furie B, Furie BC. Molecular and cellular biology of blood coagulation. N Engl J Med. 1992 Mar 19;326(12):800-6. PMID:1538724 doi:http://dx.doi.org/10.1056/NEJM199203193261205
- ↑ Shikamoto Y, Morita T, Fujimoto Z, Mizuno H. Crystal structure of Mg2+- and Ca2+-bound Gla domain of factor IX complexed with binding protein. J Biol Chem. 2003 Jun 27;278(26):24090-4. Epub 2003 Apr 14. PMID:12695512 doi:10.1074/jbc.M300650200
- ↑ Huang M, Furie BC, Furie B. Crystal structure of the calcium-stabilized human factor IX Gla domain bound to a conformation-specific anti-factor IX antibody. J Biol Chem. 2004 Apr 2;279(14):14338-46. Epub 2004 Jan 13. PMID:14722079 doi:10.1074/jbc.M314011200
- ↑ Golinelli-Pimpaneau B, Gigant B, Bizebard T, Navaza J, Saludjian P, Zemel R, Tawfik DS, Eshhar Z, Green BS, Knossow M. Crystal structure of a catalytic antibody Fab with esterase-like activity. Structure. 1994 Mar 15;2(3):175-83. PMID:8069632
- ↑ Furie BC, Blumenstein M, Furie B. Metal binding sites of a gamma-carboxyglutamic acid-rich fragment of bovine prothrombin. J Biol Chem. 1979 Dec 25;254(24):12521-30. PMID:500729
- ↑ Nakagawa T, Tani M, Kita K, Ito M. Preparation of fluorescence-labeled GM1 and sphingomyelin by the reverse hydrolysis reaction of sphingolipid ceramide N-deacylase as substrates for assay of sphingolipid-degrading enzymes and for detection of sphingolipid-binding proteins. J Biochem. 1999 Sep;126(3):604-11. PMID:10467178
Proteopedia Page Contributors and Editors (what is this?)
Nadia Dorochko, Michal Harel, Alexander Berchansky, David Canner