Sandbox Reserved 991
From Proteopedia
| Line 1: | Line 1: | ||
{{Sandbox_gvsu_chm463}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | {{Sandbox_gvsu_chm463}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | ||
<Structure load='1w6k' size='350' frame='true' align='right' caption='The ribbon structure of lanosterol synthase. The product, lanosterol, is shown in the active site. A beta-octyl glucoside detergent molecule is bound on the outside portion of the protein.' scene='Insert optional scene name here' /> | <Structure load='1w6k' size='350' frame='true' align='right' caption='The ribbon structure of lanosterol synthase. The product, lanosterol, is shown in the active site. A beta-octyl glucoside detergent molecule is bound on the outside portion of the protein.' scene='Insert optional scene name here' /> | ||
| - | This is a default text for your page ''''''. Click above on '''edit this page''' to modify. Be careful with the < and > signs. | ||
| - | You may include any references to papers as in: the use of JSmol in Proteopedia <ref>DOI 10.1002/ijch.201300024</ref> or to the article describing Jmol <ref>PMID:21638687</ref> to the rescue. | ||
== Introduction == | == Introduction == | ||
| Line 47: | Line 45: | ||
Scientific publishings in which lanosterol synthase is inhibited in varying degrees have demonstrated a direct decline in both lanosterol development and HMG-CoA reductase activity. <ref>Panini SR, Gupta A, Sexton RC, Parish EJ, Rudney H (October 1987). "Regulation of sterol biosynthesis and of 3-hydroxy-3-methylglutaryl-coenzyme A reductase activity in cultured cells by progesterone". J. Biol. Chem. 262 (30): 14435–40. PMID 3667583</ref> | Scientific publishings in which lanosterol synthase is inhibited in varying degrees have demonstrated a direct decline in both lanosterol development and HMG-CoA reductase activity. <ref>Panini SR, Gupta A, Sexton RC, Parish EJ, Rudney H (October 1987). "Regulation of sterol biosynthesis and of 3-hydroxy-3-methylglutaryl-coenzyme A reductase activity in cultured cells by progesterone". J. Biol. Chem. 262 (30): 14435–40. PMID 3667583</ref> | ||
| - | |||
| - | == Structural highlights == | ||
| - | |||
| - | This is a sample scene created with SAT to <scene name="/12/3456/Sample/1">color</scene> by Group, and another to make <scene name="/12/3456/Sample/2">a transparent representation</scene> of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes. | ||
== References == | == References == | ||
<references/> | <references/> | ||
Revision as of 21:01, 23 March 2015
| This Sandbox is Reserved from 20/01/2015, through 30/04/2016 for use in the course "CHM 463" taught by Mary Karpen at the Grand Valley State University. This reservation includes Sandbox Reserved 987 through Sandbox Reserved 996. |
To get started:
More help: Help:Editing |
|
Contents |
Introduction
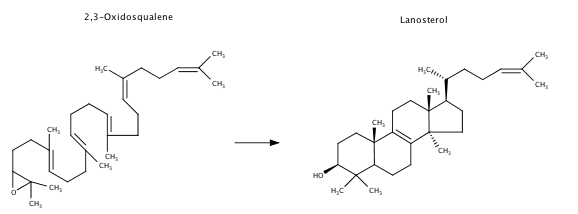
Lanosterol synthase is an enzyme found in the cholesterol biosynthesis pathway. This enzyme catalyzes the cyclization of the linear hydrocarbon, 2,3-Oxidosqualene to the four ringed . Lanosterol is a crucial precursor to cholesterol.
The gene coding for lanosterol synthase is found on chromosome 21: q22.3.
Since lanosterol synthase is involved in cholesterol biosynthesis, there is clinical relevance in using lanosterol synthase inhibitors to develop cholesterol lowering drugs. These drugs could then potentially be used in conjunction with statins.
Function
The enzyme lanosterol synthase carries out the most complex reaction of cholesterol synthesis. It takes a lengthy skinny carbon chain, 2,3-oxidosqualene, and folds it to create the cyclic lanosterol composed of four connected rings.
Cholesterol Biosynthesis
Cholesterol biosynthesis diverges from other biochemical processes using Acetyl-CoA, in the form of acetate. Acetate molecules, containing two carbons each, are condensed to form five-carbon isoprene units. These isoprene units then affix to generate a long 30 carbon molecule called squalene. Squalene will undergo an epoxidation reaction to form 2,3-oxidosqualene, the substrate for lanosterol synthase. Lanosterol continues in a 19-step pathway to become cholesterol.
Cholesterol is an important molecule that has many biological functions:
- Precursor to steroid hormones
- Formation of bile in the liver
- Incorporation into phospholipid bilayers
Mechanism
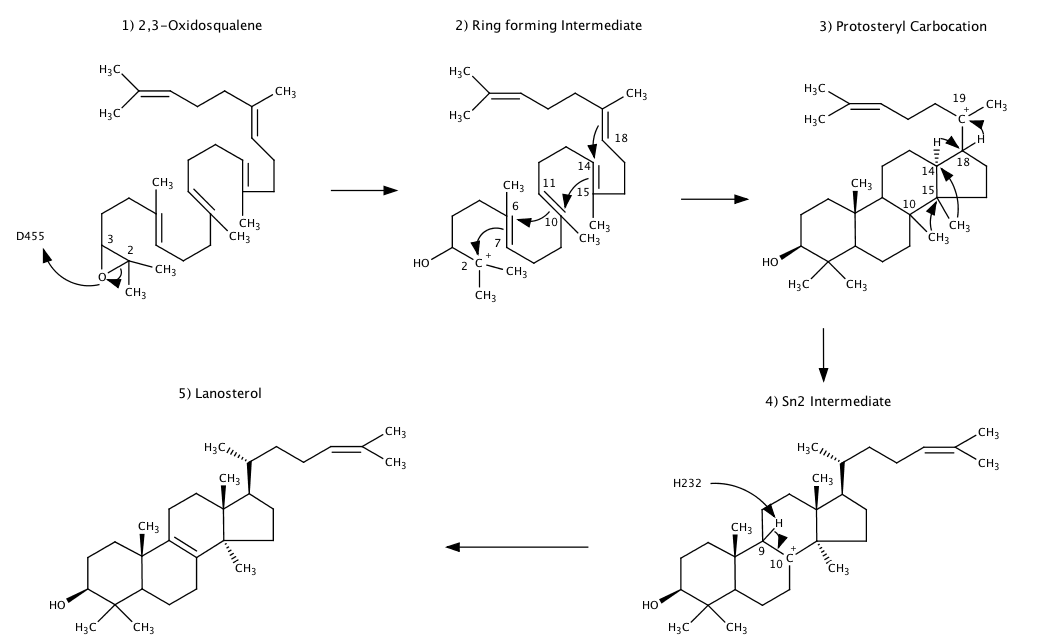
Lanosterol cyclase catalyzes the conversion of 2,3-oxidosqualene to lanosterol. Mutagenic analysis has determined that the three most residues involved in this reaction are .[1] The first step of this reaction requires the protonation of the epoxide by the the aspartic acid residue . The protonation of the oxygen causes the epoxide ring to open, forming a carbocation at postion C2 (Carbons are labeled in order of the longest chain in 2,3-Oxidosqualene). Upon formation of the carbocation, the pi electrons at adjacent double bonds transfer the positive charge across the molecule by forming four carbon-carbon single bonds between C2-C7,C6-C11, C10-15 and C14-C18. The protosteryl carbocation intermediate causes a series of proton shifts (C18 to C19, C14 to C18) and methyl transfers (C15 to C14, C10 to 15) that relocates the carbocation to C10. The last step in this reaction requires a Sn2 reaction by that forms a double bond between C9 and C10. Overall, this reaction forms 3, 6-membered rings, 1, 5-membered ring, 4 carbon-carbon bonds and 5 chiral centers.
Structure
Lanosterol synthase is a peripheral membrane protein that consists of 732 amino acids and operates as a single subunit. It has three sets of and 25 . 50% of the residues are found in helices and 5% are found in beta sheets. The residue sequence is 36-40% identical to evolutionary ancestors in plants and yeast. [2] The hydrophobic and membrane portion of the protein is bound to in the structure shown above. BOG detergent is used experimentally to isolate the protein from the membrane. [3]
Disease
Lanosterol Synthase Inhibitors as Cholesterol Lowering Drugs: There has been increased awareness surrounding the use of lanosterol synthase inhibitors as drugs to reduce LDL (low-density lipoproteins) to aid in the treatment of atherosclerosis. The commonly prescribed statin drugs currently used to lower LDL (bad blood cholesterol) while less effectively increasing HDL (high-density lipoproteins), a good blood cholesterol, function by inhibiting the activity of HMG-CoA reductase. [4] Since the lanosterol synthase enzyme catalyzes the creation of precursors upstream of cholesterol, statins could adversely affect the levels of intermediates essential for other biosynthesis pathways. So due to lanosterol synthase having a stronger association to cholesterol biosynthesis than HMG-CoA reductase, it can be considered a useful drug target. [5]
Scientific publishings in which lanosterol synthase is inhibited in varying degrees have demonstrated a direct decline in both lanosterol development and HMG-CoA reductase activity. [6]
References
- ↑ Corey EJ, Cheng CH, Baker CH, Matsuda SPT, Li D, Song X (February 1997). "Studies on the Substrate Binding Segments and Catalytic Action of Lanosterol Synthase. Affinity Labeling with Carbocations Derived from Mechanism-Based Analogs of 2, 3-Oxidosqualene and Site-Directed Mutagenesis Probes". J. Am. Chem. Soc. 119 (6): 1289–96.
- ↑ Baker, C. H., Matsuda, S. P., Liu, D. R., & Corey, E. J. (1995, August 4). Molecular cloning of the human gene encoding lanosterol synthase from a liver cDNA library. Biochemical and Biophysical Research Communications, 213(1), 154-160. Retrieved February 24, 2015, from PubMed (7639730)
- ↑ Thoma, R., Schulz-Gasch, T., D'Arcy, B., Benz, J., Aebi, J., Dehmlow, H., & Hennig, M. (2004, November 4). Insight into steroid scaffold formation from the structure of human oxidosqualene cyclase. Nature, 432(7013). doi:10.1038/nature02993
- ↑ Panini SR, Gupta A, Sexton RC, Parish EJ, Rudney H (October 1987). "Regulation of sterol biosynthesis and of 3-hydroxy-3-methylglutaryl-coenzyme A reductase activity in cultured cells by progesterone". J. Biol. Chem. 262 (30): 14435–40. PMID 3667583
- ↑ Telford DE, Lipson SM, Barrett PH, et al. (December 2005). "A novel inhibitor of oxidosqualene:lanosterol cyclase inhibits very low-density lipoprotein apolipoprotein B100 (apoB100) production and enhances low-density lipoprotein apoB100 catalbolism through marked reduction in hepatic cholesterol content". Arterioscler Thromb Vasc Biol 25 (12): 2608–14. doi:10.1161/01.ATV.0000189158.28455.94. PMID 16210564
- ↑ Panini SR, Gupta A, Sexton RC, Parish EJ, Rudney H (October 1987). "Regulation of sterol biosynthesis and of 3-hydroxy-3-methylglutaryl-coenzyme A reductase activity in cultured cells by progesterone". J. Biol. Chem. 262 (30): 14435–40. PMID 3667583