We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1084
From Proteopedia
(Difference between revisions)
| Line 33: | Line 33: | ||
HDAC8 is a part of the class I HDAC isozymes. This class is involved in expression of [http://en.wikipedia.org/wiki/P21 p21], a cyclin-dependent kinase inhibitor <ref>Blagosklonny, M. V. et al. Histone deacetylase inhibitors all induce p21 but differentially cause tubulin acetylation, mitotic arrest, and cytotoxicity. Mol. Cancer Ther. 1, 937–941 (2002).</ref>. The p21 proteins main job is to inhibit uncontrolled cell proliferation. A mutation in [http://proteopedia.org/wiki/index.php/P53 p53] gene has been reported in many cancer patients <ref name="Yan">Yan, W. et al. Histone deacetylase inhibitors suppress mutant p53 transcription via histone deacetylase 8. Oncogene 32, 599–609 (2013).</ref>. HDAC8 regulates the expression of both the wild type and the mutant form of p53 <ref name="Yan" />.Inhibition of HDACs provides an anticancer effect <ref>Dokmanovic, M., Clarke, C. & Marks, P. A. Histone Deacetylase Inhibitors: Overview and Perspectives. Mol Cancer Res 5, 981–989 (2007).</ref>. However, a pan-HDAC inhibitor usually shows considerable side effects in a clinical setting. This is probably because multiple HDAC isozymes are involved in several vital cellular processes. Isozyme selective inhibitors are likely to have fewer side effects as compared to a pan-inhibitor. They induce growth inhibition, dedifferentiation, and even cell death in cancer cells <ref>Bolden, J. E., Peart, M. J. & Johnstone, R. W. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov 5, 769–784 (2006).</ref>. It is widely known that a pan-HDAC inhibitor significantly affects the acetylation status of histones as well as several non-histone proteins, such as HSP90, p53 and others. The therapeutic potential of an HDAC8 selective activator for the treatment of various forms of cancers has recently emerged. In the above case the expression of the tumor suppressor protein (p53) is reportedly suppressed by HDAC8 <ref name="Yan" />. | HDAC8 is a part of the class I HDAC isozymes. This class is involved in expression of [http://en.wikipedia.org/wiki/P21 p21], a cyclin-dependent kinase inhibitor <ref>Blagosklonny, M. V. et al. Histone deacetylase inhibitors all induce p21 but differentially cause tubulin acetylation, mitotic arrest, and cytotoxicity. Mol. Cancer Ther. 1, 937–941 (2002).</ref>. The p21 proteins main job is to inhibit uncontrolled cell proliferation. A mutation in [http://proteopedia.org/wiki/index.php/P53 p53] gene has been reported in many cancer patients <ref name="Yan">Yan, W. et al. Histone deacetylase inhibitors suppress mutant p53 transcription via histone deacetylase 8. Oncogene 32, 599–609 (2013).</ref>. HDAC8 regulates the expression of both the wild type and the mutant form of p53 <ref name="Yan" />.Inhibition of HDACs provides an anticancer effect <ref>Dokmanovic, M., Clarke, C. & Marks, P. A. Histone Deacetylase Inhibitors: Overview and Perspectives. Mol Cancer Res 5, 981–989 (2007).</ref>. However, a pan-HDAC inhibitor usually shows considerable side effects in a clinical setting. This is probably because multiple HDAC isozymes are involved in several vital cellular processes. Isozyme selective inhibitors are likely to have fewer side effects as compared to a pan-inhibitor. They induce growth inhibition, dedifferentiation, and even cell death in cancer cells <ref>Bolden, J. E., Peart, M. J. & Johnstone, R. W. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov 5, 769–784 (2006).</ref>. It is widely known that a pan-HDAC inhibitor significantly affects the acetylation status of histones as well as several non-histone proteins, such as HSP90, p53 and others. The therapeutic potential of an HDAC8 selective activator for the treatment of various forms of cancers has recently emerged. In the above case the expression of the tumor suppressor protein (p53) is reportedly suppressed by HDAC8 <ref name="Yan" />. | ||
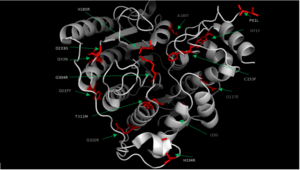
| - | In some cases of [http://en.wikipedia.org/wiki/Cornelia_de_Lange_Syndrome Cornelia de Lange syndrome] (CLdS), the cohesion acetylation cycle is impaired due to mutations in HDAC8<ref name="Mut">Deardorff, M. A. et al. HDAC8 mutations in Cornelia de Lange syndrome affect the cohesin acetylation cycle. Nature 489, 313–317 (2012).</ref>. The enzyme activity of all Class I HDACs has been found to be reduced in [http://en.wikipedia.org/wiki/Chronic_obstructive_pulmonary_disease Chronic Obstructive Pulmonary Disease] (COPD) <ref>Ito, K. et al. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N. Engl. J. Med. 352, 1967–1976 (2005).</ref>. Therapeutic potential of class I HDAC activators, specifically toward HDAC8, have not been well understood so far. However, there are several human diseases where an HDAC activator could be of great therapeutic benefits. In COPD and CLdS where the HDAC enzyme activity has been reported to be reduced, HDAC activators have a potential to lessen the disease conditions <ref name="Mut" />. | + | In some cases of [http://en.wikipedia.org/wiki/Cornelia_de_Lange_Syndrome Cornelia de Lange syndrome] (CLdS), the cohesion acetylation cycle is impaired due to mutations in HDAC8<ref name="Mut">Deardorff, M. A. et al. HDAC8 mutations in Cornelia de Lange syndrome affect the cohesin acetylation cycle. Nature 489, 313–317 (2012).</ref>. The enzyme activity of all Class I HDACs has been found to be reduced in [http://en.wikipedia.org/wiki/Chronic_obstructive_pulmonary_disease Chronic Obstructive Pulmonary Disease] (COPD) <ref>Ito, K. et al. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N. Engl. J. Med. 352, 1967–1976 (2005).</ref>. Therapeutic potential of class I HDAC activators, specifically toward HDAC8, have not been well understood so far. However, there are several human diseases where an HDAC activator could be of great therapeutic benefits. In COPD and CLdS where the HDAC enzyme activity has been reported to be reduced, HDAC activators have a potential to lessen the disease conditions <ref name="Mut" />.[[Image:HDAC8_Mutations_Final.png|300px|left|thumb| '''Fig. 2''' Various mutations of HDAC8 found in CdLS patients.]] |
| + | |||
== Structural highlights == | == Structural highlights == | ||
Revision as of 17:51, 23 April 2015
| This Sandbox is Reserved from 15/04/2015, through 15/06/2015 for use in the course "Protein structure, function and folding" taught by Taru Meri at the University of Helsinki. This reservation includes Sandbox Reserved 1081 through Sandbox Reserved 1090. |
To get started:
More help: Help:Editing |
Histone Deacetylase 8
| |||||||||||
References
- ↑ Ramakrishnan, V. Histone Structure and the Organization of the Nucleosome. Annual Review of Biophysics and Biomolecular Structure 26, 83–112 (1997).
- ↑ Waltregny, D. et al. Histone deacetylase HDAC8 associates with smooth muscle alpha-actin and is essential for smooth muscle cell contractility. FASEB J. 19, 966–968 (2005).
- ↑ Haberland, M., Mokalled, M. H., Montgomery, R. L. & Olson, E. N. Epigenetic control of skull morphogenesis by histone deacetylase 8. Genes Dev. 23, 1625–1630 (2009).
- ↑ Gräff, J. & Tsai, L.-H. The Potential of HDAC Inhibitors as Cognitive Enhancers. Annual Review of Pharmacology and Toxicology 53, 311–330 (2013).
- ↑ Blagosklonny, M. V. et al. Histone deacetylase inhibitors all induce p21 but differentially cause tubulin acetylation, mitotic arrest, and cytotoxicity. Mol. Cancer Ther. 1, 937–941 (2002).
- ↑ 6.0 6.1 6.2 Yan, W. et al. Histone deacetylase inhibitors suppress mutant p53 transcription via histone deacetylase 8. Oncogene 32, 599–609 (2013).
- ↑ Dokmanovic, M., Clarke, C. & Marks, P. A. Histone Deacetylase Inhibitors: Overview and Perspectives. Mol Cancer Res 5, 981–989 (2007).
- ↑ Bolden, J. E., Peart, M. J. & Johnstone, R. W. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov 5, 769–784 (2006).
- ↑ 9.0 9.1 Deardorff, M. A. et al. HDAC8 mutations in Cornelia de Lange syndrome affect the cohesin acetylation cycle. Nature 489, 313–317 (2012).
- ↑ Ito, K. et al. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N. Engl. J. Med. 352, 1967–1976 (2005).
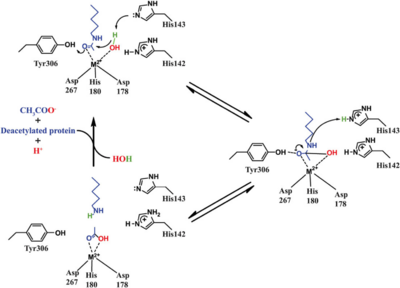
- ↑ Vannini, A. et al. Substrate binding to histone deacetylases as shown by the crystal structure of the HDAC8–substrate complex. EMBO reports 8, 879–884 (2007).
- ↑ 12.0 12.1 Somoza, J. R. et al. Structural snapshots of human HDAC8 provide insights into the class I histone deacetylases. Structure 12, 1325–1334 (2004).