Sandbox8999
From Proteopedia
| Line 11: | Line 11: | ||
Individual clathrin molecules can assemble into cage-like structures. When individual clathrin molecules come together, a lattice is formed where each lattice point is associated with the center of a triskelion (from the Greek word meaning “a three legged structure”). <ref name="harrison" /> | Individual clathrin molecules can assemble into cage-like structures. When individual clathrin molecules come together, a lattice is formed where each lattice point is associated with the center of a triskelion (from the Greek word meaning “a three legged structure”). <ref name="harrison" /> | ||
| - | [[Image:Clathrin-Cage.jpg |frame| Formation of clathrin lattice from individual clathrin monomers.]] | + | [[Image:Clathrin-Cage.jpg |frame| Formation of clathrin lattice from individual clathrin monomers.<ref name= Receptor Mediated Endocytosis.''Clathrin''.Retrived from:http://www.utm.utoronto.ca/~w3bio315/RME/clathrin.html</ref>]] |
Revision as of 23:49, 15 November 2015
Template:STRUCTURE 3lvgClathrin originates from the Latin word clāthrāre, meaning “to provide with a lattice”. Clathrin is a protein involved in receptor-mediated endocytosis. [1] It was not discovered until 1975 by Barbara Pearse, a British biological scientist. Clathrin is a protein resembling a triskelion shape and is composed of three heavy chains and three light chains which come together to form a polyhedral lattice similar to a cage.[1] The three heavy chains resemble three legs protruding from a center point. Some of the major functions of clathrin include lysosomal targeting, receptor-mediated endocytosis, and organelle biogenesis from the trans-Golgi network. [2]. The polyhedral lattice shape of clathrin largely determines its functionality, in that there are many binding sites for proteins on the heavy chains of the lattice as well. [3]
Contents |
Structure
Individual Molecule
An individual clathrin molecule is composed of three heavy chains, each being attached to a light chain. This structure is often referred to as a triskelion shape.[1] The heavy chains of clathrin are made of an amino-terminal, a beta-propellor domain containing multiple repeats of the WD40 binding motif, alpha-helical zig-zags close to 30 amino acids in length, a lengthened alpha-helix where the threefold contacts, and a carboxy-terminal.[1] The aforementioned zig-zags are what create the “leg” looking structure of the triskelion.[1] Part of the heavy chain is designated to bind to the light chains, of which there are two variations in mammals. The heavy chain and light chain bind through one long alpha helix towards the center of the protein.[1]
Lattice
Individual clathrin molecules can assemble into cage-like structures. When individual clathrin molecules come together, a lattice is formed where each lattice point is associated with the center of a triskelion (from the Greek word meaning “a three legged structure”). [1]
[[Image:Clathrin-Cage.jpg |frame| Formation of clathrin lattice from individual clathrin monomers.Cite error: Invalid <ref> tag;
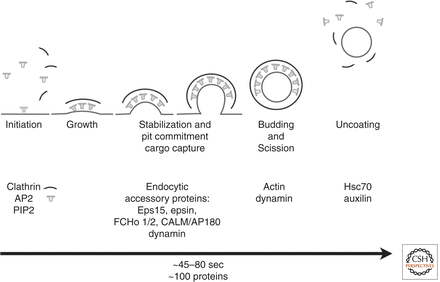
invalid names, e.g. too many Endocytic events are initiated through the activity of the clathrin coat and adaptor proteins that select the “cargo” that will be carried into the cell in vesicles.[4] At least 20 clathrin adaptors have been identified, and they all recognize and bind to membrane proteins or phospholipids that are specific for a certain organelle.[4] The formation of most cellular vesicles are facilitated by these coat-proteins like clathrin.[4] Cells endocytose vesicles from the plasma membrane to perform housekeeping tasks such as taking up nutrients, importing signaling receptors, mediating immune responses, cleaning up cell debris, or providing a pathway for pathogens or toxins.[4] Clathrin uses two main pathways for endocytosis: the canonical pathway and noncanonical pathway. The canonical clathrin pathway is referred to as the clathrin coated pits and the coated vesicles of endocytosis, whereas the noncanonical clathrin pathway is related to the clathrin-actin assembles of phagocytotic processes.[1]
The Canonical Pathway
Classical endocytosis involving clathrin-coated pits and clathrin-coated vesicles is referred to as the canonical clathrin pathway.[1] Canonical clathrin-dependent pathways involve the uptake of transferrin (Tf) by transferrin receptor (TfR) and the uptake of LDL by LDL receptors (LDLR).[1] Early studies showed that LDL epidermal growth factor (Tf) bound to their specific receptors could be endocytosed within the same vesicle.[1] The receptors for these molecules are composed of tails on their cytoplasmic side that contain sequences, which determine cargo sorting and also signal for clathrin to bind.[1] Clathrin then coats these vesicles and endocytosis occurs.[1] Other pathways, such as reuptake of neurotransmitters into the synaptic cleft, are seen to have similar mechanisms to canonical clathrin pathways.[1]
The Noncanonical Pathway
The noncanonical clathrin pathway refers to clathrin-actin assemblies, which form phagocytotic vesicles in response to invading bacterium.[1] Pathogen invasion into nonphagocytic cells result in the recruitment of clathrin.[1] These pathogens invade the cell through a process called the “zippering” mechanism, in which actin filaments are utilized to surround the pathogens with the host-cell membrane.[1] Actin recruitment requires the formation of clathrin patches and thus the clathrin-assembly forms.[1] Actin is not usually needed in classical endocytosis, but it is required when the size of the cargo in the forming vesicle is too large for the vesicle to close.[1] This association between actin and clathrin is unique to this pathway and distinguishes the noncanonical from the canonical pathway.[1]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 Harrison, S. C., Kirchhausen, T., & Owen, D. (2014). Molecular structure, function, and dynamics of clathrin-mediated membrane traffic. Cold Spring Harbor Perspectives in Biology. doi: 10.1101/cshperspect.a016725
- ↑ Wakeham, D. E., et al. (2003). Clathrin self-assembly involves coordinated weak interactions favorable for cellular recognition. The Embo Journal. doi: 10.1093/emboj/cdg511
- ↑ Ungewickell, E., & Brandon, D. (1981). Assembly units of clathrin coats. Nature, 289, 420-42. doi: 10.1038/289420a0
- ↑ 4.0 4.1 4.2 4.3 Johnson, G. T., & Goodsell, D. (2007). Molecule of the Month: Clathrin. doi: 10.2210/rcsb_pdb/mom_2007_4
