This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
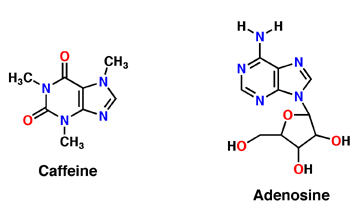
Caffeine
From Proteopedia
| Line 32: | Line 32: | ||
The A2A receptor improves the flow of blood to the heart, increasing heart rate, and additionally can lower blood pressure. When adenosine binds to the A2A receptor, cyclicAMP levels increase, and ERK1/ERK2 levels increase (Antonioli, et. al., 2013). | The A2A receptor improves the flow of blood to the heart, increasing heart rate, and additionally can lower blood pressure. When adenosine binds to the A2A receptor, cyclicAMP levels increase, and ERK1/ERK2 levels increase (Antonioli, et. al., 2013). | ||
| - | == | + | == Adenosine (A2A) Receptor == |
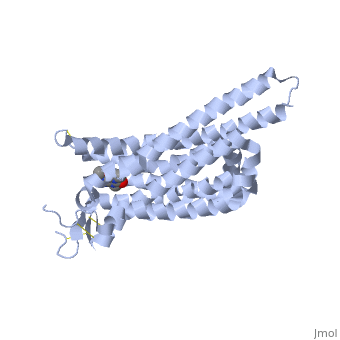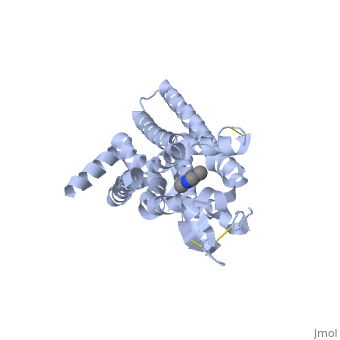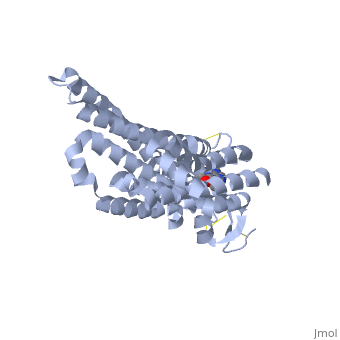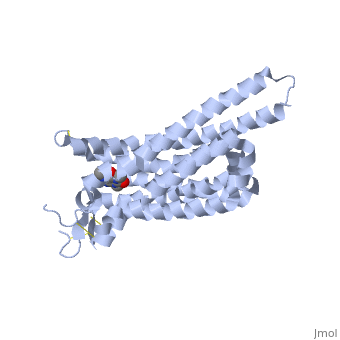
The adenosine receptor (A2A) is a G-protein coupled receptor, which is a transmembrane protein that consists of secondary structures, such as seven alpha helical domains. Inside the third and seventh transmembrane helical domains, there are hydrophobic side chains that are required for ligand recognition. The target ligand, adenosine, is a large, polar molecule that binds to the extracellular binding domain of the A2A receptor by several nonpolar interactions. To be specific, these nonpolar interactions include hydrogen bonding (11), aromatic stacking interactions (1), and many van der Waals interactions (Xu et. al, 2011). To avoid the steric interactions between the ribose ring of adenosine and the tryptophan of the enzyme binding pocket, these nonpolar interactions cause conformational changes within the binding cavity, and cause an internal rotation and tilt of the seventh helical domain (Xu et. al, 2011). Other molecules, such as caffeine can also bind to these adenosine receptors. When caffeine binds to this receptor, it inhibits adenosine from binding to the extracellular binding domain of the A2A receptor. | The adenosine receptor (A2A) is a G-protein coupled receptor, which is a transmembrane protein that consists of secondary structures, such as seven alpha helical domains. Inside the third and seventh transmembrane helical domains, there are hydrophobic side chains that are required for ligand recognition. The target ligand, adenosine, is a large, polar molecule that binds to the extracellular binding domain of the A2A receptor by several nonpolar interactions. To be specific, these nonpolar interactions include hydrogen bonding (11), aromatic stacking interactions (1), and many van der Waals interactions (Xu et. al, 2011). To avoid the steric interactions between the ribose ring of adenosine and the tryptophan of the enzyme binding pocket, these nonpolar interactions cause conformational changes within the binding cavity, and cause an internal rotation and tilt of the seventh helical domain (Xu et. al, 2011). Other molecules, such as caffeine can also bind to these adenosine receptors. When caffeine binds to this receptor, it inhibits adenosine from binding to the extracellular binding domain of the A2A receptor. | ||
Revision as of 17:55, 20 November 2015
Caffeine (Trimethylxanthine)
| |||||||||||
References
Antonioli, Luca, Corrado Blandizzi, Pal Pacher, and Gyorgy Haskó. "Adensoine and Adenosine Receptors." Nature.com. Nature Publishing Group, 2013. Web. 16 Nov. 2015.
"ADORA2B Adenosine A2b Receptor [ Homo Sapiens (human) ]." NCIB. N.p., n.d. Web. 16 Nov. 2015.
"ADORA3 Adenosine A3 Receptor [ Homo Sapiens (human) ]." NCBI. N.p., n.d. Web. 16 Nov. 2015.
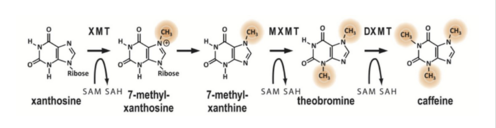
Denoeud, F., L. Carretero-Paulet, A. Dereeper, G. Droc, R. Guyot, M. Pietrella, C. Zheng, A. Alberti, F. Anthony, G. Aprea, J.-M. Aury, P. Bento, M. Bernard, S. Bocs, C. Campa, A. Cenci, M.-C. Combes, D. Crouzillat, C. Da Silva, L. Daddiego, F. De Bellis, S. Dussert, O. Garsmeur, T. Gayraud, V. Guignon, K. Jahn, V. Jamilloux, T. Joet, K. Labadie, T. Lan, J. Leclercq, M. Lepelley, T. Leroy, L.-T. Li, P. Librado, L. Lopez, A. Munoz, B. Noel, A. Pallavicini, G. Perrotta, V. Poncet, D. Pot, Priyono, M. Rigoreau, M. Rouard, J. Rozas, C. Tranchant-Dubreuil, R. Vanburen, Q. Zhang, A. C. Andrade, X. Argout, B. Bertrand, A. De Kochko, G. Graziosi, R. J. Henry, Jayarama, R. Ming, C. Nagai, S. Rounsley, D. Sankoff, G. Giuliano, V. A. Albert, P. Wincker, and P. Lashermes. "The Coffee Genome Provides Insight into the Convergent Evolution of Caffeine Biosynthesis." Science 345.6201 (2014): 1181-184.
Doré, A. S. et al. Structure of the adenosine A2A receptor in complex with ZM241385 and the xanthines XAC and caffeine. Structure 19, 1283–1293 (2011)
Mitchell, Elizabeth. Caffeine: Convergently Evolved or Creatively Provided. Digital image. Answersingenesis. N.p., 20 Sept. 2014. Web. <https://answersingenesis.org/evidence-for-creation/design-in-nature/caffeine-convergently-evolved-creatively-provided/>.
Oslen, N.L. "Caffeine Consumption Habits and Perceptions among University of New Hampshire Students" University of New Hampshire Scholars' Repository 103.1 (2013): Print.
Xanthine. Digital image. LookForDiagnosis. N.p., Sept. 2014. Web. <http://www.lookfordiagnosis.com/mesh_info.php?term=Xanthine&lang=1>.
Xu, Fei, Huizian Wu, Vsevolod Katritch, Gye Won Han, Kenneth A. Jacobson, Zhan-Guo Gao, Vadim Cherezov, and Raymond C. Stevens. "Structure of an Agonist-Bound Human A2A Adenosine Receptor." (n.d.): n. pag. Web. 8 Nov. 2015.
Xu, Fei, and Raymond C. Stevens. “Trapping Small Caffeine in a Large GPCR Pocket.” Structure (London, England : 1993) 19.9 (2011): 1204–1207. PMC. Web. 17 Nov. 2015.
Proteopedia Page Contributors and Editors (what is this?)
Mackenzie Kincer, Sidiqullah Halim, Alexander Berchansky, Michal Harel