Caffeine
From Proteopedia
| Line 32: | Line 32: | ||
The A2A receptor improves the flow of blood to the heart, increasing heart rate, and additionally can lower blood pressure. When adenosine binds to the A2A receptor, cyclicAMP levels increase, and ERK1/ERK2 levels increase (Antonioli, et. al., 2013). | The A2A receptor improves the flow of blood to the heart, increasing heart rate, and additionally can lower blood pressure. When adenosine binds to the A2A receptor, cyclicAMP levels increase, and ERK1/ERK2 levels increase (Antonioli, et. al., 2013). | ||
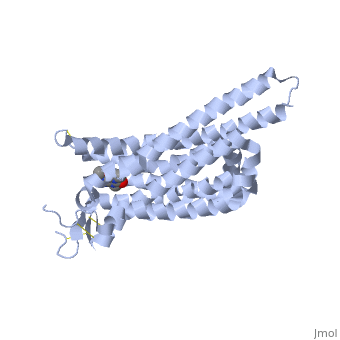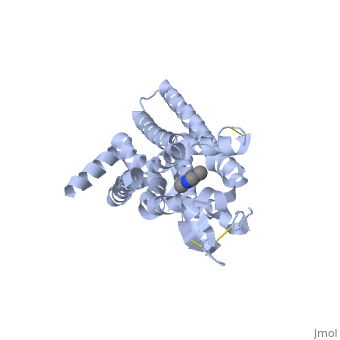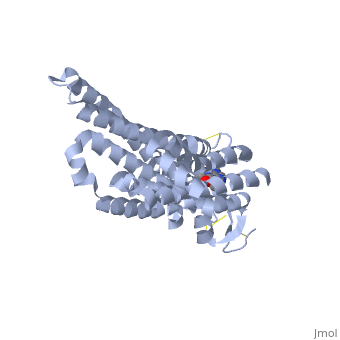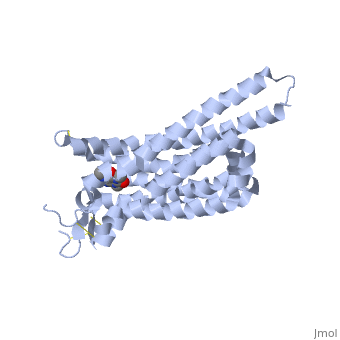
| - | == Adenosine (A2A) Receptor == | + | == Structure of Adenosine (A2A) Receptor == |
The adenosine receptor (A2A) is a G-protein coupled receptor, which is a transmembrane protein that consists of secondary structures, such as seven alpha helical domains. Inside the third and seventh transmembrane helical domains, there are hydrophobic side chains that are required for ligand recognition. The target ligand, adenosine, is a large, polar molecule that binds to the extracellular binding domain of the A2A receptor by several nonpolar interactions. To be specific, these nonpolar interactions include hydrogen bonding (11), aromatic stacking interactions (1), and many van der Waals interactions (Xu et. al, 2011). To avoid the steric interactions between the ribose ring of adenosine and the tryptophan of the enzyme binding pocket, these nonpolar interactions cause conformational changes within the binding cavity, and cause an internal rotation and tilt of the seventh helical domain (Xu et. al, 2011). Other molecules, such as caffeine can also bind to these adenosine receptors. When caffeine binds to this receptor, it inhibits adenosine from binding to the extracellular binding domain of the A2A receptor. | The adenosine receptor (A2A) is a G-protein coupled receptor, which is a transmembrane protein that consists of secondary structures, such as seven alpha helical domains. Inside the third and seventh transmembrane helical domains, there are hydrophobic side chains that are required for ligand recognition. The target ligand, adenosine, is a large, polar molecule that binds to the extracellular binding domain of the A2A receptor by several nonpolar interactions. To be specific, these nonpolar interactions include hydrogen bonding (11), aromatic stacking interactions (1), and many van der Waals interactions (Xu et. al, 2011). To avoid the steric interactions between the ribose ring of adenosine and the tryptophan of the enzyme binding pocket, these nonpolar interactions cause conformational changes within the binding cavity, and cause an internal rotation and tilt of the seventh helical domain (Xu et. al, 2011). Other molecules, such as caffeine can also bind to these adenosine receptors. When caffeine binds to this receptor, it inhibits adenosine from binding to the extracellular binding domain of the A2A receptor. | ||
| Line 38: | Line 38: | ||
== How Caffeine (Trimethylxanthine) Binds == | == How Caffeine (Trimethylxanthine) Binds == | ||
| - | A2A is a transmembrane G protein in humans. Trimethylxanthine has a highly water soluble and thus when present in the system, interacts with the A2A receptor. In order for Trimethylxantine to bind to the receptor, the third and seventh transmembrane helical domains need to recognize the ligand. Trimethylxanthine can then bind. Trimethylxanthine, due to its similar structure and its purine alkaloid structure, can bind in place of adenosine. This binding will change the shape, but not initiate the cascade of downstream effects that adenosine does, like opening of ion channels and slowing its activity. The concentrate of free adenosine increases extracellularly, when trimethylxanthine is bound. | + | A2A is a transmembrane G protein in humans. Trimethylxanthine has a highly water soluble and thus when present in the system, interacts with the A2A receptor. In order for Trimethylxantine to bind to the receptor, the third and seventh transmembrane helical domains need to recognize the ligand. Trimethylxanthine can then bind. Trimethylxanthine, due to its similar structure and its purine alkaloid structure, can bind in place of adenosine. This binding will change the shape, but not initiate the cascade of downstream effects that adenosine does, like opening of ion channels and slowing its activity. The concentrate of free adenosine increases extracellularly, when trimethylxanthine is bound (Xu and Stevens, 2011). |
| - | cAMP levels increase when adenosine is bound and are not effected when caffeine is bound. ERK1 and ERK2 are kinases, modify serine and threonine, of the GMGC group that regulation of cell growth and differentiation, and if adenosine was bound, this cascade of events would occur, but when Trimethylxanthine is bound, this regulation does not occur. | + | cAMP levels increase when adenosine is bound and are not effected when caffeine is bound. ERK1 and ERK2 are kinases, modify serine and threonine, of the GMGC group that regulation of cell growth and differentiation, and if adenosine was bound, this cascade of events would occur, but when Trimethylxanthine is bound, this regulation does not occur (Xu and Stevens, 2011). |
== Conclusion == | == Conclusion == | ||
| - | Caffeine increases temporary alertness, energy, and mood. Sensitivity to caffeine is different from person to person. It will be more effective to a small individual than a larger individual. While considered safe in small quantities, caffeine can cause irritability and headaches if someone consumes over 300 mg per day. This intake can be reduced by consumption of non-caffeinated coffee, water and tea (Dore et all | + | Caffeine increases temporary alertness, energy, and mood. Sensitivity to caffeine is different from person to person. It will be more effective to a small individual than a larger individual. While considered safe in small quantities, caffeine can cause irritability and headaches if someone consumes over 300 mg per day. This intake can be reduced by consumption of non-caffeinated coffee, water and tea (Dore ''et all'' 2011). Caffeine is still largely misunderstood, and in the next few years, more studies will be done on it because it is becoming a larger factor in today’s school and work place(Olsen, 2013). |
== See Also == | == See Also == | ||
Revision as of 18:02, 20 November 2015
Caffeine (Trimethylxanthine)
| |||||||||||
References
Antonioli, Luca, Corrado Blandizzi, Pal Pacher, and Gyorgy Haskó. "Adensoine and Adenosine Receptors." Nature.com. Nature Publishing Group, 2013. Web. 16 Nov. 2015.
"ADORA2B Adenosine A2b Receptor [ Homo Sapiens (human) ]." NCIB. N.p., n.d. Web. 16 Nov. 2015.
"ADORA3 Adenosine A3 Receptor [ Homo Sapiens (human) ]." NCBI. N.p., n.d. Web. 16 Nov. 2015.
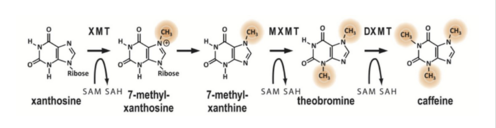
Denoeud, F., L. Carretero-Paulet, A. Dereeper, G. Droc, R. Guyot, M. Pietrella, C. Zheng, A. Alberti, F. Anthony, G. Aprea, J.-M. Aury, P. Bento, M. Bernard, S. Bocs, C. Campa, A. Cenci, M.-C. Combes, D. Crouzillat, C. Da Silva, L. Daddiego, F. De Bellis, S. Dussert, O. Garsmeur, T. Gayraud, V. Guignon, K. Jahn, V. Jamilloux, T. Joet, K. Labadie, T. Lan, J. Leclercq, M. Lepelley, T. Leroy, L.-T. Li, P. Librado, L. Lopez, A. Munoz, B. Noel, A. Pallavicini, G. Perrotta, V. Poncet, D. Pot, Priyono, M. Rigoreau, M. Rouard, J. Rozas, C. Tranchant-Dubreuil, R. Vanburen, Q. Zhang, A. C. Andrade, X. Argout, B. Bertrand, A. De Kochko, G. Graziosi, R. J. Henry, Jayarama, R. Ming, C. Nagai, S. Rounsley, D. Sankoff, G. Giuliano, V. A. Albert, P. Wincker, and P. Lashermes. "The Coffee Genome Provides Insight into the Convergent Evolution of Caffeine Biosynthesis." Science 345.6201 (2014): 1181-184.
Doré, A. S. et al. Structure of the adenosine A2A receptor in complex with ZM241385 and the xanthines XAC and caffeine. Structure 19, 1283–1293 (2011)
Mitchell, Elizabeth. Caffeine: Convergently Evolved or Creatively Provided. Digital image. Answersingenesis. N.p., 20 Sept. 2014. Web. <https://answersingenesis.org/evidence-for-creation/design-in-nature/caffeine-convergently-evolved-creatively-provided/>.
Oslen, N.L. "Caffeine Consumption Habits and Perceptions among University of New Hampshire Students" University of New Hampshire Scholars' Repository 103.1 (2013): Print.
Xanthine. Digital image. LookForDiagnosis. N.p., Sept. 2014. Web. <http://www.lookfordiagnosis.com/mesh_info.php?term=Xanthine&lang=1>.
Xu, Fei, Huizian Wu, Vsevolod Katritch, Gye Won Han, Kenneth A. Jacobson, Zhan-Guo Gao, Vadim Cherezov, and Raymond C. Stevens. "Structure of an Agonist-Bound Human A2A Adenosine Receptor." (n.d.): n. pag. Web. 8 Nov. 2015.
Xu, Fei, and Raymond C. Stevens. “Trapping Small Caffeine in a Large GPCR Pocket.” Structure (London, England : 1993) 19.9 (2011): 1204–1207. PMC. Web. 17 Nov. 2015.
Proteopedia Page Contributors and Editors (what is this?)
Mackenzie Kincer, Sidiqullah Halim, Alexander Berchansky, Michal Harel