We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox 4465
From Proteopedia
(Difference between revisions)
| Line 3: | Line 3: | ||
=='''Calmodulin'''== | =='''Calmodulin'''== | ||
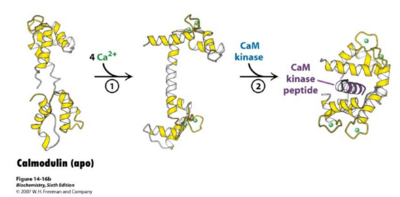
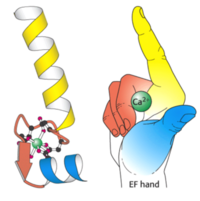
| - | Calmodulin (CaM), short for calcium modulated protein, is a small calcium binding protein with a three dimensional structure that allows calcium ions to come into the cells. Calcium is | + | Calmodulin (CaM), short for calcium modulated protein, is a small calcium binding protein with a three dimensional structure that allows calcium ions to come into the cells. Calcium is an essential mineral that is needed in the human diet for proper functioning of neurons to forming strong bones and can also act as a second messenger for enzymes and proteins. Calmodulin is known to be involved in various Ca2+ - dependent signal transduction pathways, the protein act as a Ca2+ detector, and the protein is involved with regulating protein-kinases <ref>Eldik, L., & Watterson, D. (1998). Calmodulin and signal transduction</ref>. |
| - | Its importance can be exemplified by the fact that the protein | + | Its' importance can be exemplified by the fact that the protein is known to be highly conserved in eukaryotes. Highly conserved structures that do not undergo significant evolutionary changes imply that the structure is mandatory for cell or organism survival and that any mutations in the genetic sequence that codes for the protein would be deleterious. The function of calmodulin is typically studied using yeast as a model organism. This is done for a variety of reasons, including the fact that yeast has a fully annotated genome with human homologues for genes associated with their ion channels, yeast is fast growing and yeast are heat stable<ref>Wolfe, D. M. D. M. (2006). Channeling studies in yeast: Yeast as a model for channelopathies?</ref>. Due to its’ ability to be easily manipulated, yeast can continue to be used to gather more information on calmodulins’ structure and function. |
| Line 11: | Line 11: | ||
== Calmodulin in the body == | == Calmodulin in the body == | ||
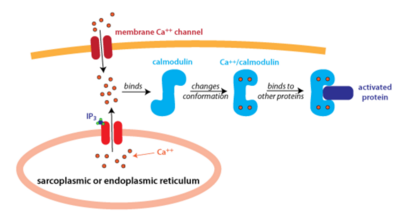
| - | Calmodulin is located and used ubiquitously by cells, but is especially prevalent in brain and muscle tissue. It has also been found human serum, breast milk, urine, and saliva<ref>MacNeil S., Dawson RA., Crocker G., Barton CH., Hanford L., McGurk MR., and Munro DS., (1988). Extracellular calmodulin and its association with epidermal growth factor in normal human body fluids.</ref>. Calmodulin in the cell is mainly localized within organelles and binds to Calcium. Calcium binding then promotes the phosphorylation of protein kinases and activation of other proteins to begin signal transduction for a variety of different pathways, mainly different forms of cell signaling. The phosphorylation of these protein-kinases occurs when Ca2+ reach about 1000 nM and initiates a rapid signaling pathway<ref>doi:10.1038/35036035</ref>. | + | Calmodulin is located and used ubiquitously by cells, but is especially prevalent in brain and muscle tissue. It has also been found in human serum, breast milk, urine, and saliva<ref>MacNeil S., Dawson RA., Crocker G., Barton CH., Hanford L., McGurk MR., and Munro DS., (1988). Extracellular calmodulin and its association with epidermal growth factor in normal human body fluids.</ref>. Calmodulin in the cell is mainly localized within organelles and binds to Calcium. Calcium binding then promotes the phosphorylation of protein kinases and activation of other proteins to begin signal transduction for a variety of different pathways, mainly different forms of cell signaling. The phosphorylation of these protein-kinases occurs when Ca2+ reach about 1000 nM and initiates a rapid signaling pathway<ref>doi:10.1038/35036035</ref>. |
[[Image:Calmodulin_fig_2.png| thumb|left|400px| '''Figure 2: An Overview of Calmodulin Pathway''' Calmodulin binds to 4 Calcium Ions and Undergoes Conformational Changes]] | [[Image:Calmodulin_fig_2.png| thumb|left|400px| '''Figure 2: An Overview of Calmodulin Pathway''' Calmodulin binds to 4 Calcium Ions and Undergoes Conformational Changes]] | ||
Revision as of 22:48, 7 December 2015
Calmodulin
| |||||||||||
Bibliography
- ↑ Eldik, L., & Watterson, D. (1998). Calmodulin and signal transduction
- ↑ Wolfe, D. M. D. M. (2006). Channeling studies in yeast: Yeast as a model for channelopathies?
- ↑ MacNeil S., Dawson RA., Crocker G., Barton CH., Hanford L., McGurk MR., and Munro DS., (1988). Extracellular calmodulin and its association with epidermal growth factor in normal human body fluids.
- ↑ Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000 Oct;1(1):11-21. PMID:11413485 doi:http://dx.doi.org/10.1038/35036035
- ↑ Huang X, Liu Y, Wang R, Zhong X, Liu Y, Koop A, Chen SR, Wagenknecht T, Liu Z. Two potential calmodulin-binding sequences in the ryanodine receptor contribute to a mobile, intra-subunit calmodulin-binding domain. J Cell Sci. 2013 Oct 1;126(Pt 19):4527-35. doi: 10.1242/jcs.133454. Epub 2013 Jul, 18. PMID:23868982 doi:http://dx.doi.org/10.1242/jcs.133454
- ↑ Wriggers W, Mehler E, Pitici F, Weinstein H, Schulten K. Structure and dynamics of calmodulin in solution. Biophys J. 1998 Apr;74(4):1622-39. doi: 10.1016/S0006-3495(98)77876-2. PMID:9545028 doi:http://dx.doi.org/10.1016/S0006-3495(98)77876-2
- ↑ Lai M, Brun D, Edelstein SJ, Le Novere N. Modulation of calmodulin lobes by different targets: an allosteric model with hemiconcerted conformational transitions. PLoS Comput Biol. 2015 Jan 22;11(1):e1004063. doi: 10.1371/journal.pcbi.1004063. , eCollection 2015 Jan. PMID:25611683 doi:http://dx.doi.org/10.1371/journal.pcbi.1004063
- ↑ Chan KF, Chen WH. High performance capillary electrophoresis of calmodulin. Electrophoresis. 1990 Jan;11(1):15-8. PMID:2108018 doi:http://dx.doi.org/10.1002/elps.1150110104
- ↑ Bagchi IC, Huang QH, Means AR. Identification of amino acids essential for calmodulin binding and activation of smooth muscle myosin light chain kinase. J Biol Chem. 1992 Feb 15;267(5):3024-9. PMID:1737757
- ↑ Joseph JD, Means AR. Calcium binding is required for calmodulin function in Aspergillus nidulans. Eukaryot Cell. 2002 Feb;1(1):119-25. doi: 10.1128/ec.01.1.119-125.2002. PMID:12455978 doi:http://dx.doi.org/10.1128/ec.01.1.119-125.2002
- ↑ Lewit-Bentley, A., & Rèty S. (2000). EF-hand calcium-binding proteins. The Journal of current opinion in structural biology, 10(6), 637-643.doi:10.1016/S0959-440X(00)00142-1
- ↑ Racioppi L, Noeldner PK, Lin F, Arvai S, Means AR. Calcium/calmodulin-dependent protein kinase kinase 2 regulates macrophage-mediated inflammatory responses. J Biol Chem. 2012 Mar 30;287(14):11579-91. doi: 10.1074/jbc.M111.336032. Epub, 2012 Feb 14. PMID:22334678 doi:http://dx.doi.org/10.1074/jbc.M111.336032
- ↑ Ui-Tei, K., Nagano, M., Sato, S., & Miyata, Y. (2000). Calmodulin-dependent and -independent apoptosis in cell of a drosophila neuronal cell line