User:Wade Cook/Sandbox 1
From Proteopedia
| Line 16: | Line 16: | ||
[[Image:Active_site_(new).png]] | [[Image:Active_site_(new).png]] | ||
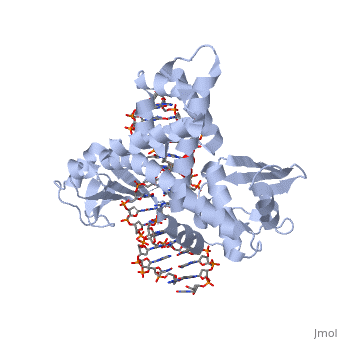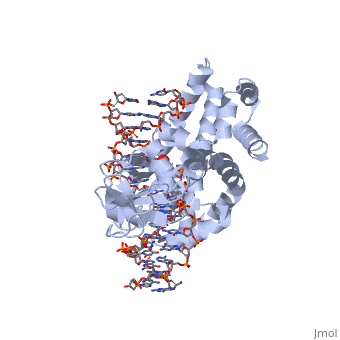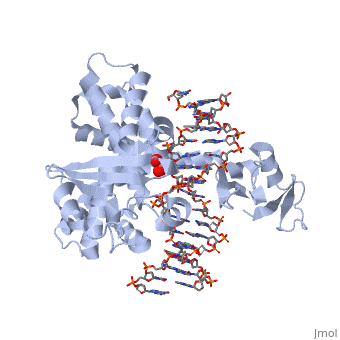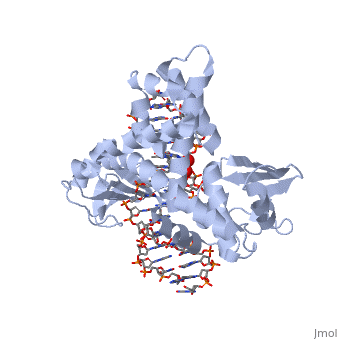
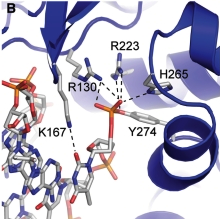
| - | In the figure above, [A] shows the amino acid residues located within the active site of eukaryotic topoisomerase 1B. [B] shows the amino acid residues located within the active site of viral topoisomerase 1B. Both active sites contain similar residues and are highly conserved among the different species. Researchers have proven that an asparagine residue can replace the histidine residue in the active site of the viral topoisomerase, and the enzyme will still undergo the same cleavage mechanism. This suggests that the same cleavage and reliagation mechanisms are the same in all topoisomerase 1B’s <ref name="Baker" />.Type IB topoisomerase is a key target for research against the spread of smallpox because it is integral for the viruses replication process. The replication of smallpox is complicated since it doesn’t hijack the host’s genetic machinery to reproduce, this makes the disease highly virulent, and hard to specifically target for elimination by antiviral drugs <ref name="Baker" />. | + | In the figure above, [A] shows the amino acid residues located within the active site of eukaryotic topoisomerase 1B. [B] shows the amino acid residues located within the active site of viral topoisomerase 1B. Both active sites contain similar residues and are highly conserved among the different species. Researchers have proven that an asparagine residue can replace the <scene name='71/716538/Histidine/1'>histidine</scene> residue in the active site of the viral topoisomerase, and the enzyme will still undergo the same cleavage mechanism. This suggests that the same cleavage and reliagation mechanisms are the same in all topoisomerase 1B’s <ref name="Baker" />.Type IB topoisomerase is a key target for research against the spread of smallpox because it is integral for the viruses replication process. The replication of smallpox is complicated since it doesn’t hijack the host’s genetic machinery to reproduce, this makes the disease highly virulent, and hard to specifically target for elimination by antiviral drugs <ref name="Baker" />. |
== Relevance == | == Relevance == | ||
Current revision
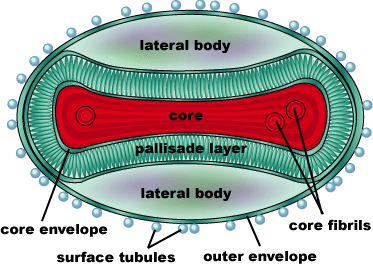
Smallpox (Variola Virus) - Topoisomerase 1B
Smallpox is an acute, highly contagious disease, which causes disfiguring and febrile rash-like illness, which has no known cure. According to some health experts, smallpox was responsible for more deaths than all other infectious diseases combined thus far in the world's history [1]. The disease causes high morbidity and mortality and led to the deaths of approximately 500 million people in the 20th century alone. With so many people affected, in an intensive public health vaccination campaign was initiated by the World Health Organization (WHO) to eradicate smallpox as a human disease in the 1960’s. There were two forms of the disease worldwide: Variola major, the deadly disease, and Variola minor, a much milder form [2]. Although naturally occurring smallpox no longer exists, there are concerns that the variola virus could be used as an agent of bioterrorism or biowarfare. As a result, it is critical to understand the molecular dynamics and virulence factors in order to prepare for a potential epidemic and to prevent the devastating consequences [2].
| |||||||||||
References
- ↑ 1.0 1.1 Smith, K. “Smallpox. can we still learn from the journey to eradication?” Indian Journal Of Medicine. 137.5 (2013): 895-899.
- ↑ 2.0 2.1 2.2 “Smallpox.” Center for Disease Control and Prevention. CDC, n.d. Web. 28 Oct. 2015. <http://www.bt.cdc.gov/agent/smallpox/index.asp>
- ↑ Shubhash, and Parija. "Poxviruses." Textbook of Microbiology and Immunity. Ed. Chandra. India: Elsevior, 2009. 484. Print.
- ↑ 4.0 4.1 4.2 Berwald, Juli. "Variola Virus." Encyclopedia of Espionage, Intelligence, and Security. 2004.Encyclopedia.com. 28 Oct. 2015 <http://www.encyclopedia.com>
- ↑ "PENN Medicine News: Penn Researchers Determine Structure of Smallpox Virus Protein Bound to DNA." PENN Medicine News: Penn Researchers Determine Structure of Smallpox Virus Protein Bound to DNA. PENN Medicine, 4 Aug. 2006. Web. 28 Oct. 2015. <http://www.uphs.upenn.edu/news/News_Releases/aug06/smlpxenz.htm>
- ↑ Perry, Kay, Young Hwang, Frederic D. Bushman, and Gregory D. Van Duyne. "Insights from the Structure of a Smallpox Virus Topoisomerase-DNA Transition State Mimic." Structure (London, England : 1993). U.S. National Library of Medicine, n.d. Web. 28 Oct. 2015. <http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2822398/>
- ↑ Minkah, Nana et al. “Variola Virus Topoisomerase: DNA Cleavage Specificity and Distribution of Sites in Poxvirus Genomes.” Virology 365.1 (2007): 60–69.PMC. Web. 16 Nov. 2015
- ↑ 8.0 8.1 8.2 Baker, Nicole M., Rakhi Rajan, and Alfonso Mondragón. “Structural Studies of Type I Topoisomerases.” Nucleic Acids Research 37.3 (2009): 693–701. PMC. Web. 16 Nov. 2015>