We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Jonathan Lloyd/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 8: | Line 8: | ||
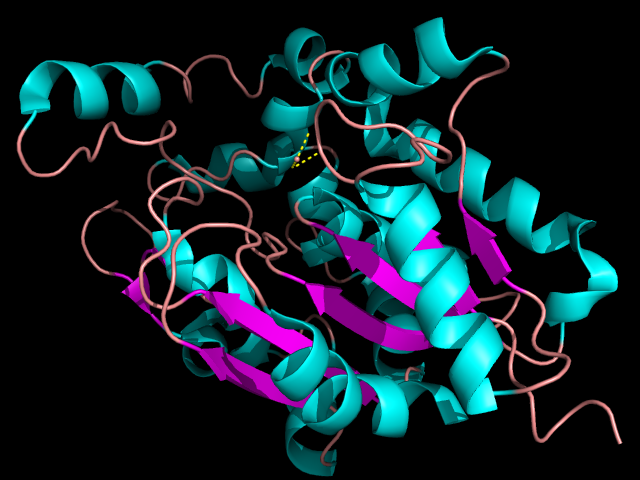
Since much is unknown about ATAD2b we look to its paralogue ATAD2a which has been the focus of many studies. ATAD2a and ATAD2b both share the AAA and bromodomain, in which they are 97% and 74% identical respectively. identical and Based on the observed high sequence similarity with well characterized bromodomains, the ATAD2b bromodomain is expected to recognize acetylated lysine. However since no tests have yet to be done on this additional studies are needed to identify the exact modifications that the ATAD2b bromodomain may recognize. Throughout all bromodomains it seems as though there are very important residues that reside in the binding pocket, Tyr760, Tyr802 and Asn803. These three amino acids are highly conserved and thus must have a major impact in the binding pocket, they are most likely the key to bromodomains binding to acetylated lysine modifications. | Since much is unknown about ATAD2b we look to its paralogue ATAD2a which has been the focus of many studies. ATAD2a and ATAD2b both share the AAA and bromodomain, in which they are 97% and 74% identical respectively. identical and Based on the observed high sequence similarity with well characterized bromodomains, the ATAD2b bromodomain is expected to recognize acetylated lysine. However since no tests have yet to be done on this additional studies are needed to identify the exact modifications that the ATAD2b bromodomain may recognize. Throughout all bromodomains it seems as though there are very important residues that reside in the binding pocket, Tyr760, Tyr802 and Asn803. These three amino acids are highly conserved and thus must have a major impact in the binding pocket, they are most likely the key to bromodomains binding to acetylated lysine modifications. | ||
| - | + | [[Image:pymol.png]] | |
== Sequence Analysis == | == Sequence Analysis == | ||
Revision as of 18:26, 9 December 2015
ATAD2b
| |||||||||||