We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Epidermal Growth Factor Receptor
From Proteopedia
(Difference between revisions)
| Line 5: | Line 5: | ||
==EGFR and Lung Cancer== | ==EGFR and Lung Cancer== | ||
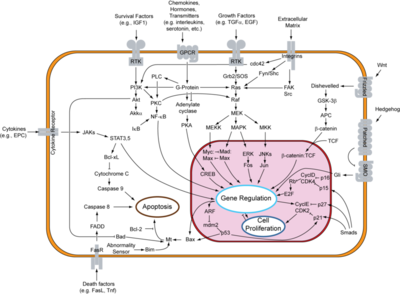
| - | [[Image:800px-Signal_transduction_v1.png|thumb|left|400px|Signal Transduction Pathways Including that of EGFR]]The binding of epidermal growth factor (EGF) to epidermal growth factor receptor (EGFR) results in the activation of its protein tyrosine kinase activity due to an induced dimerization of the receptor(1). This binding of EGF to the EGF receptor is an essential part of the signal transduction pathway that occurs in cells. This binding results eventually in cell division. Problems in this pathway can result in cancerous cells and tumors that eventually can lead to death. Therefore, understanding all parts of signal transduction is essential for understanding the cause of cancer and possible treatments of it. When EGF binds to EGFR, it results in an activation of the receptor which causes a chain of signaling events that leads to cell proliferation. This occurs by transmission of the signal across the plasma membrane because EGFR is a plasma membrane protein and in this sense a transmembrane receptor (1). Although it is present in normal cells, EGFR is overexpressed in many types of tumor cell lines, and its activation also plays an important role in resistance to chemotherapy and radiation treatment which makes treatment of cancer even more difficult(2). Since overexpression of EGFR is an attribute of some cancers, it is a plausible idea to find EGFR inhibitors to decrease the activity of EGFR and therefore inhibit cell proliferation and help control the growth of cancer and tumors. EGFR inhibitors have been developed and seem to contribute at times to the regression of tumors (2). | + | [[Image:800px-Signal_transduction_v1.png|thumb|left|400px|Signal Transduction Pathways Including that of EGFR]] |
| + | {{Clear}} | ||
| + | The binding of epidermal growth factor (EGF) to epidermal growth factor receptor (EGFR) results in the activation of its protein tyrosine kinase activity due to an induced dimerization of the receptor(1). This binding of EGF to the EGF receptor is an essential part of the signal transduction pathway that occurs in cells. This binding results eventually in cell division. Problems in this pathway can result in cancerous cells and tumors that eventually can lead to death. Therefore, understanding all parts of signal transduction is essential for understanding the cause of cancer and possible treatments of it. When EGF binds to EGFR, it results in an activation of the receptor which causes a chain of signaling events that leads to cell proliferation. This occurs by transmission of the signal across the plasma membrane because EGFR is a plasma membrane protein and in this sense a transmembrane receptor (1). Although it is present in normal cells, EGFR is overexpressed in many types of tumor cell lines, and its activation also plays an important role in resistance to chemotherapy and radiation treatment which makes treatment of cancer even more difficult(2). Since overexpression of EGFR is an attribute of some cancers, it is a plausible idea to find EGFR inhibitors to decrease the activity of EGFR and therefore inhibit cell proliferation and help control the growth of cancer and tumors. EGFR inhibitors have been developed and seem to contribute at times to the regression of tumors (2). | ||
== '''EGFR and Lung Cancers of "Never Smokers"''' == | == '''EGFR and Lung Cancers of "Never Smokers"''' == | ||
Revision as of 13:22, 10 February 2016
| |||||||||||
3D Structures of Epidermal Growth Factor Receptor
Updated on 10-February-2016
Additional Resources
For additional information, see: Cancer
References
1.Sherrill, Jennifer M., and Jack Kyte. "Activation of Epidermal Growth Factor Receptor by Epidermal Growth Factor†." Biochemistry 35 (1996): 5705-718. Print.
2.Herbst, R. S. "Review of epidermal growth factor receptor biology." Int J Radiat Oncol Biol Phys. 59 (2994). Print.
3.Pao, William, and Vincent Miller. "EGF receptor gene mutations are common in lung cancers from ‘‘never smokers’’ and are associated with sensitivity of tumors to gefitinib and erlotinib." PNAS 101 (2004). Print.
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, David Canner, Alexander Berchansky, Joel L. Sussman, Jaime Prilusky