We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1172
From Proteopedia
(Difference between revisions)
| Line 7: | Line 7: | ||
Lysophosphatidic Acid Receptor 1 (commonly referred to as LPA<sub>1</sub>) is a [[G protein-coupled receptor]] and one of 6 different LPA receptors (LPA<sub>1</sub>-LPA<sub>6</sub>) that binds the phospholipid derivative [https://en.wikipedia.org/wiki/Lysophosphatidic_acid lysophosphatidic acid (LPA)], a signaling molecule that acts as a potent [https://en.wikipedia.org/wiki/Mitogen mitogen] upon binding to one of its six receptors.<ref name="regpeps">PMID: 26091040</ref> LPA<sub>1</sub> is part of the larger [http://jb.oxfordjournals.org/content/131/6/767 EDG receptor family] which includes the more widely known sphingosine 1-phopshate receptors. | Lysophosphatidic Acid Receptor 1 (commonly referred to as LPA<sub>1</sub>) is a [[G protein-coupled receptor]] and one of 6 different LPA receptors (LPA<sub>1</sub>-LPA<sub>6</sub>) that binds the phospholipid derivative [https://en.wikipedia.org/wiki/Lysophosphatidic_acid lysophosphatidic acid (LPA)], a signaling molecule that acts as a potent [https://en.wikipedia.org/wiki/Mitogen mitogen] upon binding to one of its six receptors.<ref name="regpeps">PMID: 26091040</ref> LPA<sub>1</sub> is part of the larger [http://jb.oxfordjournals.org/content/131/6/767 EDG receptor family] which includes the more widely known sphingosine 1-phopshate receptors. | ||
== Structure == | == Structure == | ||
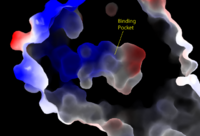
| - | [[Image:Amphbindingfinal.png| | + | [[Image:Amphbindingfinal.png|200 px|left|thumb|Figure 1: Electrostatic illustration of the amphipathic binding pocket of the LPA<sub>1</sub> receptor. This binding pocket, located in the interior of the protein, has both polar and nonpolar regions. The blue and red coloration highlight the positively and negatively charged regions, respectively, and the white color shows the nonpolar region of the binding pocket.]] |
The LPA<sub>1</sub> receptor protein is composed of 364 amino acids with a mass of approximately 41 kDa. Just as other G-protein coupled receptors, LPA<sub>1</sub> contains seven [http://kinemage.biochem.duke.edu/teaching/anatax/html/anatax.2a.html alpha helices] which make up the seven transmembrane spanning domains with three intracellular loops and three extracellular loops. The amino terminus of this protein is located on the extracellular side of the membrane, while the carboxyl terminus is located on the intracellular side of the membrane.<ref name = 'Hernández-Méndez'>Hernández-Méndez, Aurelio, Rocío Alcántara-Hernández, and J. Adolfo García-Sáinz. "Lysophosphatidic Acid LPA1-3 Receptors: Signaling, Regulation and in Silico Analysis of Their Putative Phosphorylation Sites." Receptors & Clinical Investigation Receptor Clin Invest 1.3 (2014). Web. 15 Feb. 2016.' </ref>. Within these helices is an amphipathic binding pocket which stabilizes the binding of its ligand, LPA. | The LPA<sub>1</sub> receptor protein is composed of 364 amino acids with a mass of approximately 41 kDa. Just as other G-protein coupled receptors, LPA<sub>1</sub> contains seven [http://kinemage.biochem.duke.edu/teaching/anatax/html/anatax.2a.html alpha helices] which make up the seven transmembrane spanning domains with three intracellular loops and three extracellular loops. The amino terminus of this protein is located on the extracellular side of the membrane, while the carboxyl terminus is located on the intracellular side of the membrane.<ref name = 'Hernández-Méndez'>Hernández-Méndez, Aurelio, Rocío Alcántara-Hernández, and J. Adolfo García-Sáinz. "Lysophosphatidic Acid LPA1-3 Receptors: Signaling, Regulation and in Silico Analysis of Their Putative Phosphorylation Sites." Receptors & Clinical Investigation Receptor Clin Invest 1.3 (2014). Web. 15 Feb. 2016.' </ref>. Within these helices is an amphipathic binding pocket which stabilizes the binding of its ligand, LPA. | ||
=== Key Ligand Interactions === | === Key Ligand Interactions === | ||
| - | [[Image: | + | [[Image:Ligandpocket2.png|200 px|right|thumb|Surface representation of the LPA<sub>1</sub> receptor in tan interacting with its antagonist, ON7, shown in green and red sticks.]] |
Three separate interactions with an antagonist of LPA<sub>1</sub>, ON7, help demonstrate the key interactions that stabilize the binding of the LPA phospholipid to this receptor. At the polar region of the binding pocket, the majority of this region is stabilized by <scene name='72/721543/Arg124gln125/1'>Arg124 and Gln125</scene> forming ionic and polar interactions with the carboxylic acid and the hydroxyl group of ON7.<ref name="regpeps">PMID: 26091040</ref> In addition, interplay between <scene name='72/721543/Lys39_and_glu293/4'>Glu293 and Lys39</scene> causes another stabilizing component of the ON7 antagonist. Glu293 forms polar interactions with Lys39, positioning it in close proximity to to the carboxylic acid of ON7, which then interactions with Lys39 via ionic bonding.<ref name="regpeps">PMID: 26091040</ref> While Lys39 is highly conserved among all six LPA receptors, a His40 residue is present that is specific to the LPA<sub>1</sub> receptor. <scene name='72/721543/His40/1'>His40</scene> forms both ionic and polar interactions with the carboxylic acid of ON7. The protonation of this residue has been found to greatly affect the binding affinity of LPA, and is an important link to tumor growth and survival in acidic environments.<ref name="regpeps">PMID: 26091040</ref> | Three separate interactions with an antagonist of LPA<sub>1</sub>, ON7, help demonstrate the key interactions that stabilize the binding of the LPA phospholipid to this receptor. At the polar region of the binding pocket, the majority of this region is stabilized by <scene name='72/721543/Arg124gln125/1'>Arg124 and Gln125</scene> forming ionic and polar interactions with the carboxylic acid and the hydroxyl group of ON7.<ref name="regpeps">PMID: 26091040</ref> In addition, interplay between <scene name='72/721543/Lys39_and_glu293/4'>Glu293 and Lys39</scene> causes another stabilizing component of the ON7 antagonist. Glu293 forms polar interactions with Lys39, positioning it in close proximity to to the carboxylic acid of ON7, which then interactions with Lys39 via ionic bonding.<ref name="regpeps">PMID: 26091040</ref> While Lys39 is highly conserved among all six LPA receptors, a His40 residue is present that is specific to the LPA<sub>1</sub> receptor. <scene name='72/721543/His40/1'>His40</scene> forms both ionic and polar interactions with the carboxylic acid of ON7. The protonation of this residue has been found to greatly affect the binding affinity of LPA, and is an important link to tumor growth and survival in acidic environments.<ref name="regpeps">PMID: 26091040</ref> | ||
Revision as of 13:11, 12 April 2016
| This Sandbox is Reserved from Jan 11 through August 12, 2016 for use in the course CH462 Central Metabolism taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1160 through Sandbox Reserved 1184. |
To get started:
More help: Help:Editing |
Lysophosphatidic Acid Receptor 1
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 Chrencik JE, Roth CB, Terakado M, Kurata H, Omi R, Kihara Y, Warshaviak D, Nakade S, Asmar-Rovira G, Mileni M, Mizuno H, Griffith MT, Rodgers C, Han GW, Velasquez J, Chun J, Stevens RC, Hanson MA. Crystal Structure of Antagonist Bound Human Lysophosphatidic Acid Receptor 1. Cell. 2015 Jun 18;161(7):1633-43. doi: 10.1016/j.cell.2015.06.002. PMID:26091040 doi:http://dx.doi.org/10.1016/j.cell.2015.06.002
- ↑ Hernández-Méndez, Aurelio, Rocío Alcántara-Hernández, and J. Adolfo García-Sáinz. "Lysophosphatidic Acid LPA1-3 Receptors: Signaling, Regulation and in Silico Analysis of Their Putative Phosphorylation Sites." Receptors & Clinical Investigation Receptor Clin Invest 1.3 (2014). Web. 15 Feb. 2016.'
- ↑ Yung, Y. C., N. C. Stoddard, and J. Chun. "LPA Receptor Signaling: Pharmacology, Physiology, and Pathophysiology." The Journal of Lipid Research 55.7 (2014): 1192-214. Web. 17 Feb. 2016.'
- ↑ Chun, J., Hla, T., Spiegel, S., and Moolenaar, W.H. “Lysophospholipid Receptors: Signaling and Biochemistry.” John Wiley & Sons, Inc. (2013) pp.i-xviii. 5 Feb. 2016.'
- ↑ Anliker B, Choi JW, Lin ME, Gardell SE, Rivera RR, Kennedy G, Chun J. Lysophosphatidic acid (LPA) and its receptor, LPA1 , influence embryonic schwann cell migration, myelination, and cell-to-axon segregation. Glia. 2013 Dec;61(12):2009-22. doi: 10.1002/glia.22572. Epub 2013 Sep 24. PMID:24115248 doi:http://dx.doi.org/10.1002/glia.22572
- ↑ Lin ME, Herr DR, Chun J. Lysophosphatidic acid (LPA) receptors: signaling properties and disease relevance. Prostaglandins Other Lipid Mediat. 2010 Apr;91(3-4):130-8. doi:, 10.1016/j.prostaglandins.2009.02.002. Epub 2009 Mar 4. PMID:20331961 doi:http://dx.doi.org/10.1016/j.prostaglandins.2009.02.002
- ↑ Justus CR, Dong L, Yang LV. Acidic tumor microenvironment and pH-sensing G protein-coupled receptors. Front Physiol. 2013 Dec 5;4:354. doi: 10.3389/fphys.2013.00354. PMID:24367336 doi:http://dx.doi.org/10.3389/fphys.2013.00354
- ↑ Chun, E., Thompson, A.A., Lui, W., Roth, C.B., Griffith, M.T., Katritch, V., Kunken, J., Xu, F., Cherezov, V., Hanson, M.A., and Stevens, R.C. “Fusion partner tool chest for the stabilization and crystallization of G protein-coupled receptors.” Structure 20, (2012) 967-976.'
- ↑ Van Durme, J., Horn, F., Costagliola, S., Vriend, G., and Vassart, G. “GRIS: glycoprotein-hormone receptor information system.” Mol. (2006) Endocrinol. 20, 2247-2255'