Beta Secretase (BACE1) 1SGZ
From Proteopedia
| Line 32: | Line 32: | ||
== Active Site Mechanism == | == Active Site Mechanism == | ||
| - | The mechanism of this protease activity is an acid-base reaction, common in organic chemistry. The two aspartate molecules coordinate a water molecule through hydrogen bonding. The aspartate molecule takes a hydrogen from a water molecule, which activates the water as a good nucleophile. The good water nucleophile attacks the carbonyl carbon in the peptide bond with the attack forms a tetrahedral intermediate <ref name=" | + | The mechanism of this protease activity is an acid-base reaction, common in organic chemistry. The two aspartate molecules coordinate a water molecule through hydrogen bonding. The aspartate molecule takes a hydrogen from a water molecule, which activates the water as a good nucleophile. The good water nucleophile attacks the carbonyl carbon in the peptide bond with the attack forms a tetrahedral intermediate <ref name="nine"> Golub, M. (Producer) & Golub, M. (Director). (2015). Youtube. United States: University of Rochester Introductory Biochemistry </ref>. A pair of electrons is moved to re-create a carbonyl group which breaks the peptide bond and re-generates an aspartic oxyanion <ref name="ten">Manada, N., Tanokashira, D., Hoska, A., Kametani, F., Tamoka, A,. and Araki, W. (2015). Amyloid beta-protein oligomers upregulate the beta-secretase, BACE1, through a post-translational mechanism involving its altered subcellular distribution in neurons. Molecular Brain, Vol. 8, p.1-12. 12p. doi: 10.1186/s13041-015-0163-5</ref>. No covalent bonds are formed between the beta-secretase side chains and the substrate on the enzyme and therefore the fragments can exit the active site easily. |
[[Image:BsecretaseActiveSiteMechanism.png]] | [[Image:BsecretaseActiveSiteMechanism.png]] | ||
| Line 40: | Line 40: | ||
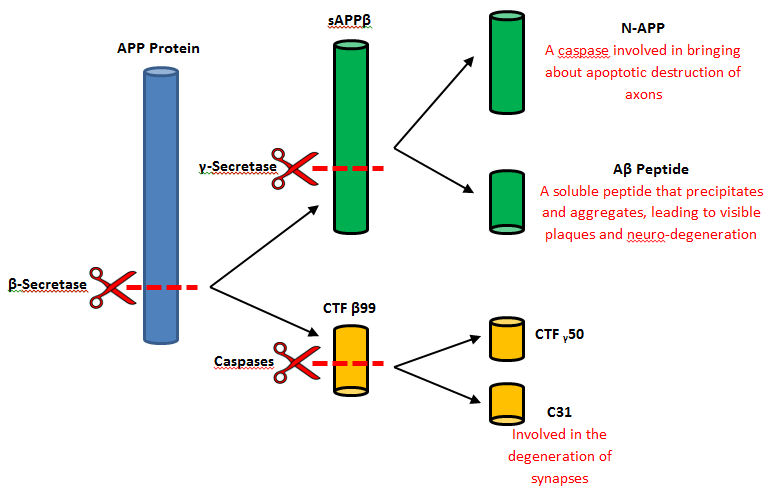
BACE-1 plays a main role for the proteolytic processing of APP. The main function is to release the extracellular material of the beta-cleaved soluble APP by the cleaving of the N-terminus of the A-beta peptide sequence while the C-terminal fragment is released by a process of gamma-secretase <ref name="six">Venugopal, C.; Demos, C. M.; Rao, K. S. J.; Pappolla, M. A.; Sambamurti, K. Beta-secretase: Structure, Function, and Evolution. CNS & Neurological Disorders Drug Targets. [Online] 2008. 7(3), 278–294. | BACE-1 plays a main role for the proteolytic processing of APP. The main function is to release the extracellular material of the beta-cleaved soluble APP by the cleaving of the N-terminus of the A-beta peptide sequence while the C-terminal fragment is released by a process of gamma-secretase <ref name="six">Venugopal, C.; Demos, C. M.; Rao, K. S. J.; Pappolla, M. A.; Sambamurti, K. Beta-secretase: Structure, Function, and Evolution. CNS & Neurological Disorders Drug Targets. [Online] 2008. 7(3), 278–294. | ||
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2921875/ | http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2921875/ | ||
| - | </ref>. However, beta-secretase malfunctions to cleave APP in a different position creating beta-amyloid. These fragments of beta-amyloid then aggregate and form harmful plaques that can spread throughout the body causing Alzheimer’s Disease. Beta-secretase also takes place in the formation of myelin sheaths in the peripheral nerve cells <ref name=" | + | </ref>. However, beta-secretase malfunctions to cleave APP in a different position creating beta-amyloid. These fragments of beta-amyloid then aggregate and form harmful plaques that can spread throughout the body causing Alzheimer’s Disease. Beta-secretase also takes place in the formation of myelin sheaths in the peripheral nerve cells <ref name="nine"> Golub, M. (Producer) & Golub, M. (Director). (2015). Youtube. United States: University of Rochester Introductory Biochemistry </ref>. |
| - | [[Image:CleavageOfBSecretase.jpg]] | + | [[Image:CleavageOfBSecretase.jpg]] <ref>JCSciphile, March - Beta-Secretase, 2014, Web, Retrieved April 5, 2016 from http://jcsciphile.com/molecule-of-the-month/march-beta-secretase/</ref>. |
| - | In a general reaction, APP is initially cleaved by alpha-secretase in a certain position every time and then by gamma-secretase. Neurons may benefits from the released fragments produced by the alpha-secretase and gamma-secretase cleavage of APP <ref name=" | + | In a general reaction, APP is initially cleaved by alpha-secretase in a certain position every time and then by gamma-secretase. Neurons may benefits from the released fragments produced by the alpha-secretase and gamma-secretase cleavage of APP <ref name="nine"> Golub, M. (Producer) & Golub, M. (Director). (2015). Youtube. United States: University of Rochester Introductory Biochemistry </ref>. However, when beta-secretase enters the equation the cleavage of APP changes positions. Once gamma-secretase has officially completed its cleavage, amyloid-beta is released into the extracellular membrane space. Amyloid-beta is most commonly found to aggregate in this area and form plaques that are extremely harmful to the human body. It is thought that this plaque build up is the initial cause of Alzheimer’s Disease <ref name="ten">Manada, N., Tanokashira, D., Hoska, A., Kametani, F., Tamoka, A,. and Araki, W. (2015). Amyloid beta-protein oligomers upregulate the beta-secretase, BACE1, through a post-translational mechanism involving its altered subcellular distribution in neurons. Molecular Brain, Vol. 8, p.1-12. 12p. doi: 10.1186/s13041-015-0163-5</ref>. |
== Associated Health Conditions == | == Associated Health Conditions == | ||
Revision as of 02:45, 13 April 2016
Beta-secretase, also known as BACE or Memapsin 2, is encoded by the gene BACE1. Beta-secretase is a proteolytic, transmembrane enzyme with two active sites on the extracellular region. It is associated with processing amyloid precursor protein (APP), which is an integral membrane protein.[1] A malfunction in the processing of APP results in the formation of the peptide amyloid beta. Amyloid-beta is a neurotoxic peptide segment that aggregates into plaques. These plaques are the primary component of plaques found in individuals with Alzheimers Disease. [2] Other biological associations of this enzyme include modulating myelination in the central and peripheral nervous systems.[3]
Enzyme Class
Beta-secretase is an enzyme that is classified as a class 3 enzyme, which are hydrolases [4]. The enzyme acts on breaking peptide bonds and therefore is also considered a peptidase and belongs to the subclass of aspartic acid endopeptidases.[5]
Structure
| |||||||||||
References
- ↑ Ghosh, A.; Kumaragurubaran, N.; Hong, L.; Koelsh, G.; Tang, J. Memapsin 2 (Beta-Secretase) Inhibitors: Drug Development. CAR Current Alzheimer Research. [Online] 2008, 5, 121–131. http://jamesmadisonva.library.ingentaconnect.com/content/ben/car/2008/00000005/00000002/art00004
- ↑ John, V.; Beck, J. P.; Bienkowski, M. J.; Sinha, S.; Heinrikson, R. L. Human β-Secretase (BACE) And BACE Inhibitors. ChemInform. [Online] 2004, 35, 4625-4630. http://pubs.acs.org/doi/pdf/10.1021/jm030247h
- ↑ Hu, X.; Hicks, C. W.; He, W.; Wong, P.; Macklin, W. B.; Trapp, B. D.; Yan, R. Bace1 Modulates Myelination in the Central and Peripheral Nervous System. Nature Neuroscience Nat Neurosci. [Online] 2006, 9, 1520–1525. http://www.nature.com/neuro/journal/v9/n12/abs/nn1797.html
- ↑ Nomenclature Committee of the International Union of Biochemistry and Molecular Biology. The Enzyme List Class 3 — Hydrolases. http://www.enzyme-database.org/downloads/ec3.pdf
- ↑ DBGET Search. KEGG ENZYME: 3.4.23.46. http://www.genome.jp/dbget-bin/www_bget?ec:3.4.23.46
- ↑ 6.0 6.1 6.2 Venugopal, C.; Demos, C. M.; Rao, K. S. J.; Pappolla, M. A.; Sambamurti, K. Beta-secretase: Structure, Function, and Evolution. CNS & Neurological Disorders Drug Targets. [Online] 2008. 7(3), 278–294. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2921875/
- ↑ 7.0 7.1 7.2 Shimizu, H.; Tosaki, A.; Kaneko, K.; Hisano, T.; Sakurai, T.; Nukina, N. Crystal Structure of an Active Form of BACE1, an Enzyme Responsible for Amyloid Beta Protein Production. Molecular and Cellular Biology [Online] 2008, 28(11), 3663-671. http://mcb.asm.org/content/28/11/3663.full.pdf+html
- ↑ Kumalo, M.; Soumendranath B.; Soliman, M. E. Investigation of Flap Flexibility of β-secretase Using Molecular Dynamic Simulations. Journal of Biomolecular Structure and Dynamics [Online] , 2015, 1-12. http://www.tandfonline.com/doi/full/10.1080/07391102.2015.1064831
- ↑ 9.0 9.1 9.2 Golub, M. (Producer) & Golub, M. (Director). (2015). Youtube. United States: University of Rochester Introductory Biochemistry
- ↑ 10.0 10.1 Manada, N., Tanokashira, D., Hoska, A., Kametani, F., Tamoka, A,. and Araki, W. (2015). Amyloid beta-protein oligomers upregulate the beta-secretase, BACE1, through a post-translational mechanism involving its altered subcellular distribution in neurons. Molecular Brain, Vol. 8, p.1-12. 12p. doi: 10.1186/s13041-015-0163-5
- ↑ Sugana, K., Padlan E., Smith, C., Carlson, W., and Davis, D. (1987). Retrieved April 5, 2016 from

- ↑ JCSciphile, March - Beta-Secretase, 2014, Web, Retrieved April 5, 2016 from http://jcsciphile.com/molecule-of-the-month/march-beta-secretase/