We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1172
From Proteopedia
(Difference between revisions)
| Line 5: | Line 5: | ||
== Introduction == | == Introduction == | ||
| - | Lysophosphatidic Acid Receptor 1 (commonly referred to as LPA<sub>1</sub>) is a [[G protein-coupled receptor]] and one of 6 different LPA receptors (LPA<sub>1</sub>-LPA<sub>6</sub>). These receptors bind the phospholipid derivative [https://en.wikipedia.org/wiki/Lysophosphatidic_acid lysophosphatidic acid (LPA)], a signaling molecule that acts as a potent [https://en.wikipedia.org/wiki/Mitogen mitogen] upon binding to one of its six receptors.<ref name="regpeps">PMID: 26091040</ref> LPA<sub>1</sub> is part of the larger [http://jb.oxfordjournals.org/content/131/6/767 EDG receptor family] which includes the more widely | + | Lysophosphatidic Acid Receptor 1 (commonly referred to as LPA<sub>1</sub>) is a [[G protein-coupled receptor]] and one of 6 different LPA receptors (LPA<sub>1</sub>-LPA<sub>6</sub>). These receptors bind the phospholipid derivative [https://en.wikipedia.org/wiki/Lysophosphatidic_acid lysophosphatidic acid (LPA)], a signaling molecule that acts as a potent [https://en.wikipedia.org/wiki/Mitogen mitogen] upon binding to one of its six receptors.<ref name="regpeps">PMID: 26091040</ref> LPA<sub>1</sub> is part of the larger [http://jb.oxfordjournals.org/content/131/6/767 EDG receptor family], which includes the more widely studied sphingosine 1-phopshate receptors. |
== Structure == | == Structure == | ||
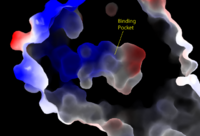
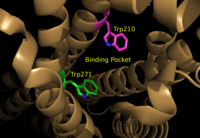
[[Image:Amphbindingfinal.png|200 px|left|thumb|Figure 1: Electrostatic illustration of the amphipathic binding pocket of the LPA<sub>1</sub> receptor. This binding pocket was revealed by cutting away the exterior or the protein. This binding pocket, located in the interior of the protein, has both polar and nonpolar regions. The blue and red coloration highlight the positively and negatively charged regions, respectively, and the white color shows the nonpolar region of the binding pocket.]] | [[Image:Amphbindingfinal.png|200 px|left|thumb|Figure 1: Electrostatic illustration of the amphipathic binding pocket of the LPA<sub>1</sub> receptor. This binding pocket was revealed by cutting away the exterior or the protein. This binding pocket, located in the interior of the protein, has both polar and nonpolar regions. The blue and red coloration highlight the positively and negatively charged regions, respectively, and the white color shows the nonpolar region of the binding pocket.]] | ||
Revision as of 01:15, 14 April 2016
| This Sandbox is Reserved from Jan 11 through August 12, 2016 for use in the course CH462 Central Metabolism taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1160 through Sandbox Reserved 1184. |
To get started:
More help: Help:Editing |
Lysophosphatidic Acid Receptor 1
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 Chrencik JE, Roth CB, Terakado M, Kurata H, Omi R, Kihara Y, Warshaviak D, Nakade S, Asmar-Rovira G, Mileni M, Mizuno H, Griffith MT, Rodgers C, Han GW, Velasquez J, Chun J, Stevens RC, Hanson MA. Crystal Structure of Antagonist Bound Human Lysophosphatidic Acid Receptor 1. Cell. 2015 Jun 18;161(7):1633-43. doi: 10.1016/j.cell.2015.06.002. PMID:26091040 doi:http://dx.doi.org/10.1016/j.cell.2015.06.002
- ↑ Hernández-Méndez, Aurelio, Rocío Alcántara-Hernández, and J. Adolfo García-Sáinz. "Lysophosphatidic Acid LPA1-3 Receptors: Signaling, Regulation and in Silico Analysis of Their Putative Phosphorylation Sites." Receptors & Clinical Investigation Receptor Clin Invest 1.3 (2014). Web. 15 Feb. 2016.'
- ↑ Yung, Y. C., N. C. Stoddard, and J. Chun. "LPA Receptor Signaling: Pharmacology, Physiology, and Pathophysiology." The Journal of Lipid Research 55.7 (2014): 1192-214. Web. 17 Feb. 2016.'
- ↑ Chun, J., Hla, T., Spiegel, S., and Moolenaar, W.H. “Lysophospholipid Receptors: Signaling and Biochemistry.” John Wiley & Sons, Inc. (2013) pp.i-xviii. 5 Feb. 2016.'
- ↑ Anliker B, Choi JW, Lin ME, Gardell SE, Rivera RR, Kennedy G, Chun J. Lysophosphatidic acid (LPA) and its receptor, LPA1 , influence embryonic schwann cell migration, myelination, and cell-to-axon segregation. Glia. 2013 Dec;61(12):2009-22. doi: 10.1002/glia.22572. Epub 2013 Sep 24. PMID:24115248 doi:http://dx.doi.org/10.1002/glia.22572
- ↑ Chun, E., Thompson, A.A., Lui, W., Roth, C.B., Griffith, M.T., Katritch, V., Kunken, J., Xu, F., Cherezov, V., Hanson, M.A., and Stevens, R.C. “Fusion partner tool chest for the stabilization and crystallization of G protein-coupled receptors.” Structure 20, (2012) 967-976.'
- ↑ Van Durme, J., Horn, F., Costagliola, S., Vriend, G., and Vassart, G. “GRIS: glycoprotein-hormone receptor information system.” Mol. (2006) Endocrinol. 20, 2247-2255'
- ↑ Lin ME, Herr DR, Chun J. Lysophosphatidic acid (LPA) receptors: signaling properties and disease relevance. Prostaglandins Other Lipid Mediat. 2010 Apr;91(3-4):130-8. doi:, 10.1016/j.prostaglandins.2009.02.002. Epub 2009 Mar 4. PMID:20331961 doi:http://dx.doi.org/10.1016/j.prostaglandins.2009.02.002
- ↑ Justus CR, Dong L, Yang LV. Acidic tumor microenvironment and pH-sensing G protein-coupled receptors. Front Physiol. 2013 Dec 5;4:354. doi: 10.3389/fphys.2013.00354. PMID:24367336 doi:http://dx.doi.org/10.3389/fphys.2013.00354