Sandbox reserved 1169
From Proteopedia
(Difference between revisions)
| Line 11: | Line 11: | ||
== Structure == | == Structure == | ||
=== Overall Structure === | === Overall Structure === | ||
| - | [[Image:Nuerotensin membrane.jpg |100 px|left|thumb|Figure 1: Neurotensin Incorporation in Membrane. This image depicts the spanning of the membrane made by NTSR1 and illustrates the need for transduction from its extracellular binding site to the intracellular region. | + | [[Image:Nuerotensin membrane.jpg |100 px|left|thumb|Figure 1: Neurotensin Incorporation in Membrane. This image depicts the spanning of the membrane made by NTSR1 and illustrates the need for transduction from its extracellular binding site to the intracellular region. (PubMed).]] |
Like other G protein-coupled receptors, the neurotensin receptor is composed of 3 distinct regions. An extracellular binding site where neurotensin binds and causes a conformational change of the protein. A region containing <scene name='72/727765/Overall_structure/4'>7 transmembrane alpha helices</scene> (PDB code:[http://www.rcsb.org/pdb/explore/explore.do?structureId=4GRV 4GRV)] that transduce the signal from the extracellular side of the cell membrane to the intracellular side. Lastly, an intracellular region, that when activated by a conformational change in the protein, activates a [https://en.wikipedia.org/wiki/G_protein G protein] associated with this receptor. Currently no crystal structures of the inactive form of the neurotensin receptor available. Without a representation of the inactive form, the conformational changes caused by agonist binding are still not completely known. | Like other G protein-coupled receptors, the neurotensin receptor is composed of 3 distinct regions. An extracellular binding site where neurotensin binds and causes a conformational change of the protein. A region containing <scene name='72/727765/Overall_structure/4'>7 transmembrane alpha helices</scene> (PDB code:[http://www.rcsb.org/pdb/explore/explore.do?structureId=4GRV 4GRV)] that transduce the signal from the extracellular side of the cell membrane to the intracellular side. Lastly, an intracellular region, that when activated by a conformational change in the protein, activates a [https://en.wikipedia.org/wiki/G_protein G protein] associated with this receptor. Currently no crystal structures of the inactive form of the neurotensin receptor available. Without a representation of the inactive form, the conformational changes caused by agonist binding are still not completely known. | ||
=== Neurotensin Binding Site === | === Neurotensin Binding Site === | ||
| Line 22: | Line 22: | ||
Sodium ions are a negative [https://en.wikipedia.org/wiki/Allosteric_regulation allosteric] inhibitor to the binding of the neurotensin [https://en.wikipedia.org/wiki/Agonist agonist] to the binding site on the neurotensin receptor. Sodium's binding causes for the receptor to favor its inactive state. Asp113 of the highly conserved D/RY motif and Asn365 of the highly conserved NPxxY motif form a substantial hydrogen bonding network with T156 and S362.<ref name="Krumm"/> This hydrogen bonding network prevents the incorporation of the sodium ion by collapsing upon itself and filling the sodium binding pocket. Trp321 also works to inhibit the incorporation of the sodium ion by capping off the sodium binding pocket to not allow sodium to enter from the top. Trp321 uses van der Walls interactions to place it in the conformation necessary to activate the G-protein that is associated with this receptor. | Sodium ions are a negative [https://en.wikipedia.org/wiki/Allosteric_regulation allosteric] inhibitor to the binding of the neurotensin [https://en.wikipedia.org/wiki/Agonist agonist] to the binding site on the neurotensin receptor. Sodium's binding causes for the receptor to favor its inactive state. Asp113 of the highly conserved D/RY motif and Asn365 of the highly conserved NPxxY motif form a substantial hydrogen bonding network with T156 and S362.<ref name="Krumm"/> This hydrogen bonding network prevents the incorporation of the sodium ion by collapsing upon itself and filling the sodium binding pocket. Trp321 also works to inhibit the incorporation of the sodium ion by capping off the sodium binding pocket to not allow sodium to enter from the top. Trp321 uses van der Walls interactions to place it in the conformation necessary to activate the G-protein that is associated with this receptor. | ||
==Clinical Relevance== | ==Clinical Relevance== | ||
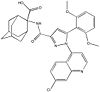
| - | NTSR1 is commonly expressed in various invasive [https://en.wikipedia.org/wiki/Cancer cancer] cell lines making it a promising cancer drug target. It is prevalent in [https://en.wikipedia.org/wiki/Colorectal_cancer colon cancer] [https://en.wikipedia.org/wiki/Adenocarcinoma adenocarcinoma], but is not found in adult colon cell types.<ref name="Valerie">PMID:21903767</ref> NTSR1 is also found in aggressive [https://en.wikipedia.org/wiki/Prostate_cancer prostate cancer] cells, but not [https://en.wikipedia.org/wiki/Epithelium epithelial] prostate cells. In prostate cancer cells, binding of NTS results in [https://en.wikipedia.org/wiki/Mitogen-activated_protein_kinase mitogen-activated protein kinase (PKB)], [https://en.wikipedia.org/wiki/Phosphoinositide_3-kinase phosphoinositide-3 kinase (PI-3K)], [https://en.wikipedia.org/wiki/Epidermal_growth_factor_receptor epidermal growth factor receptor (EGFR)], [https://en.wikipedia.org/wiki/Proto-oncogene_tyrosine-protein_kinase_Src SRC], and [https://en.wikipedia.org/wiki/STAT5 STAT5] phosphorylation.<ref name="Valerie"/> These all result in increased DNA synthesis, [https://en.wikipedia.org/wiki/Cell_growth cell proliferation], and survival. Inhibition of NTSR1 and its downstream signaling represents a target for [https://en.wikipedia.org/wiki/Radiation_therapy radiotherapy], which uses radiation to target malignant cells. [[Image: Meclinerant.jpg |100 px|left|thumb|Meclinerant: An inhibitor of NTSR1 found to enhance selectivity of radiotherapy in cancer treatment ]]NTSR1 can be inhibited by agonist [https://en.wikipedia.org/wiki/Meclinertant meclinertant] which inhibits proliferation and prosurvival of cancer cells. Combination treatment of radiation and meclinerant provides selective treatment of cancer cells over normal cells, indicating the need for clinical trials of this approach. <ref name="Kisfalvi">PMID:19679549</ref> | + | NTSR1 is commonly expressed in various invasive [https://en.wikipedia.org/wiki/Cancer cancer] cell lines making it a promising cancer drug target. It is prevalent in [https://en.wikipedia.org/wiki/Colorectal_cancer colon cancer] [https://en.wikipedia.org/wiki/Adenocarcinoma adenocarcinoma], but is not found in adult colon cell types.<ref name="Valerie">PMID:21903767</ref> NTSR1 is also found in aggressive [https://en.wikipedia.org/wiki/Prostate_cancer prostate cancer] cells, but not [https://en.wikipedia.org/wiki/Epithelium epithelial] prostate cells. In prostate cancer cells, binding of NTS results in [https://en.wikipedia.org/wiki/Mitogen-activated_protein_kinase mitogen-activated protein kinase (PKB)], [https://en.wikipedia.org/wiki/Phosphoinositide_3-kinase phosphoinositide-3 kinase (PI-3K)], [https://en.wikipedia.org/wiki/Epidermal_growth_factor_receptor epidermal growth factor receptor (EGFR)], [https://en.wikipedia.org/wiki/Proto-oncogene_tyrosine-protein_kinase_Src SRC], and [https://en.wikipedia.org/wiki/STAT5 STAT5] phosphorylation.<ref name="Valerie"/> These all result in increased DNA synthesis, [https://en.wikipedia.org/wiki/Cell_growth cell proliferation], and survival. Inhibition of NTSR1 and its downstream signaling represents a target for [https://en.wikipedia.org/wiki/Radiation_therapy radiotherapy], which uses radiation to target malignant cells. [[Image: Meclinerant.jpg |100 px|left|thumb|Meclinerant: An inhibitor of NTSR1 found to enhance selectivity of radiotherapy in cancer treatment (PubMed).]]NTSR1 can be inhibited by agonist [https://en.wikipedia.org/wiki/Meclinertant meclinertant] which inhibits proliferation and prosurvival of cancer cells. Combination treatment of radiation and meclinerant provides selective treatment of cancer cells over normal cells, indicating the need for clinical trials of this approach. <ref name="Kisfalvi">PMID:19679549</ref> |
Revision as of 04:11, 18 April 2016
Neurotensin Receptor (NTSR1)
References
- ↑ Millar RP, Newton CL. The year in G protein-coupled receptor research. Mol Endocrinol. 2010 Jan;24(1):261-74. Epub 2009 Dec 17. PMID:20019124 doi:10.1210/me.2009-0473
- ↑ Gui X, Carraway RE. Enhancement of jejunal absorption of conjugated bile acid by neurotensin in rats. Gastroenterology. 2001 Jan;120(1):151-60. PMID:11208724
- ↑ Selivonenko VG. [The interrelationship between electrolytes and phase analysis of systole in toxic goiter]. Probl Endokrinol (Mosk). 1975 Jan-Feb;21(1):19-23. PMID:1173461
- ↑ Fang Y, Lahiri J, Picard L. G protein-coupled receptor microarrays for drug discovery. Drug Discov Today. 2004 Dec 15;9(24 Suppl):S61-7. PMID:23573662
- ↑ 5.0 5.1 White JF, Noinaj N, Shibata Y, Love J, Kloss B, Xu F, Gvozdenovic-Jeremic J, Shah P, Shiloach J, Tate CG, Grisshammer R. Structure of the agonist-bound neurotensin receptor. Nature. 2012 Oct 25;490(7421):508-13. doi: 10.1038/nature11558. Epub 2012 Oct 10. PMID:23051748 doi:http://dx.doi.org/10.1038/nature11558
- ↑ Carraway R, Leeman SE. The isolation of a new hypotensive peptide, neurotensin, from bovine hypothalami. J Biol Chem. 1973 Oct 10;248(19):6854-61. PMID:4745447
- ↑ Kitabgi P. Neurotensin modulates dopamine neurotransmission at several levels along brain dopaminergic pathways. Neurochem Int. 1989;14(2):111-9. PMID:20504406
- ↑ Mustain WC, Rychahou PG, Evers BM. The role of neurotensin in physiologic and pathologic processes. Curr Opin Endocrinol Diabetes Obes. 2011 Feb;18(1):75-82. doi:, 10.1097/MED.0b013e3283419052. PMID:21124211 doi:http://dx.doi.org/10.1097/MED.0b013e3283419052
- ↑ Vincent JP, Mazella J, Kitabgi P. Neurotensin and neurotensin receptors. Trends Pharmacol Sci. 1999 Jul;20(7):302-9. PMID:10390649
- ↑ 10.0 10.1 10.2 White JF, Noinaj N, Shibata Y, Love J, Kloss B, Xu F, Gvozdenovic-Jeremic J, Shah P, Shiloach J, Tate CG, Grisshammer R. Structure of the agonist-bound neurotensin receptor. Nature. 2012 Oct 25;490(7421):508-13. doi: 10.1038/nature11558. Epub 2012 Oct 10. PMID:23051748 doi:http://dx.doi.org/10.1038/nature11558
- ↑ Katritch V, Fenalti G, Abola EE, Roth BL, Cherezov V, Stevens RC. Allosteric sodium in class A GPCR signaling. Trends Biochem Sci. 2014 May;39(5):233-44. doi: 10.1016/j.tibs.2014.03.002. Epub , 2014 Apr 21. PMID:24767681 doi:http://dx.doi.org/10.1016/j.tibs.2014.03.002
- ↑ 12.0 12.1 Valerie NC, Casarez EV, Dasilva JO, Dunlap-Brown ME, Parsons SJ, Amorino GP, Dziegielewski J. Inhibition of neurotensin receptor 1 selectively sensitizes prostate cancer to ionizing radiation. Cancer Res. 2011 Nov 1;71(21):6817-26. doi: 10.1158/0008-5472.CAN-11-1646. Epub, 2011 Sep 8. PMID:21903767 doi:http://dx.doi.org/10.1158/0008-5472.CAN-11-1646
- ↑ Kisfalvi K, Eibl G, Sinnett-Smith J, Rozengurt E. Metformin disrupts crosstalk between G protein-coupled receptor and insulin receptor signaling systems and inhibits pancreatic cancer growth. Cancer Res. 2009 Aug 15;69(16):6539-45. doi: 10.1158/0008-5472.CAN-09-0418. PMID:19679549 doi:http://dx.doi.org/10.1158/0008-5472.CAN-09-0418