Sandbox Reserved 1172
From Proteopedia
(Difference between revisions)
| Line 24: | Line 24: | ||
=== Sphingosine 1-Phosphate Receptor === | === Sphingosine 1-Phosphate Receptor === | ||
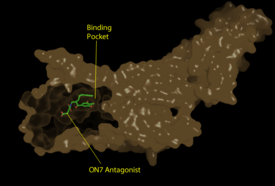
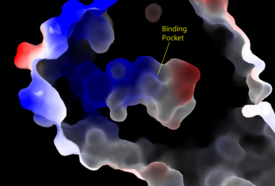
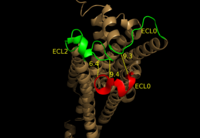
| - | LPA<sub>1</sub> belongs to the EDG (endothelial differentiation gene) family of [https://en.wikipedia.org/wiki/Lysophospholipid_receptor lysophospholipid receptors]. This family also includes the [https://en.wikipedia.org/wiki/S1PR1 sphingosine 1-phosphate receptor 1] (S1P<sub>1</sub>), which shares many structural similarities to LPA<sub>1</sub>. In fact, the transmembrane regions share a sequence identity of 41%. <ref name = 'Chun, E.'>Chun, E., Thompson, A.A., Lui, W., Roth, C.B., Griffith, M.T., Katritch, V., Kunken, J., Xu, F., Cherezov, V., Hanson, M.A., and Stevens, R.C. “Fusion partner tool chest for the stabilization and crystallization of G protein-coupled receptors.” Structure 20, (2012) 967-976.' </ref> A defining difference between these two receptors is their mode of ligand access to the binding site. The hydrophobic [https://en.wikipedia.org/wiki/Sphingosine-1-phosphate S1P ligand] enters S1P<sub>1</sub> via the membrane. LPA<sub>1</sub> differs and utilizes an extracellular opening that allows LPA access from the extracellular space (Figure | + | LPA<sub>1</sub> belongs to the EDG (endothelial differentiation gene) family of [https://en.wikipedia.org/wiki/Lysophospholipid_receptor lysophospholipid receptors]. This family also includes the [https://en.wikipedia.org/wiki/S1PR1 sphingosine 1-phosphate receptor 1] (S1P<sub>1</sub>), which shares many structural similarities to LPA<sub>1</sub>. In fact, the transmembrane regions share a sequence identity of 41%. <ref name = 'Chun, E.'>Chun, E., Thompson, A.A., Lui, W., Roth, C.B., Griffith, M.T., Katritch, V., Kunken, J., Xu, F., Cherezov, V., Hanson, M.A., and Stevens, R.C. “Fusion partner tool chest for the stabilization and crystallization of G protein-coupled receptors.” Structure 20, (2012) 967-976.' </ref> A defining difference between these two receptors is their mode of ligand access to the binding site. The hydrophobic [https://en.wikipedia.org/wiki/Sphingosine-1-phosphate S1P ligand] enters S1P<sub>1</sub> via the membrane. LPA<sub>1</sub> differs and utilizes an extracellular opening that allows LPA access from the extracellular space (Figure 4). <ref name="regpeps">PMID: 26091040</ref> Structural evidence for this altered ligand binding pathway includes global changes in the positioning of the extracellular loops (ECL) and transmembrane helices (TM). Specifically, a slight divergence of <scene name='72/721543/Tmvii_and_tmi/1'>TMI</scene>, which is positioned 3 Å closer to TMVII compared to S1P<sub>1</sub>, and a repositioning of <scene name='72/721543/Ecl_regions/1'>ECL3</scene>, resulting in a divergence of 8 Å from S1P<sub>1</sub> result in ligand access via the extracellular space. <ref name="regpeps">PMID: 26091040</ref> This narrowing of the gap between TMI and TMVII blocks membrane ligand access in LPA<sub>1</sub>, while the greater distance between ECL3 and the other extracellular loops promotes extracellular access for LPA<sub>1</sub>. Additionally, ECL0 is helical in S1P<sub>1</sub>, but <scene name='72/721543/Ecl02ndstructure/1'>lacks secondary structure</scene> in LPA<sub>1</sub>. This increased flexibility that results from ECL0 lack of secondary structure in LPA<sub>1</sub> further promotes favorable LPA access to the binding pocket from the extracellular space. <ref name="regpeps">PMID: 26091040</ref> |
| Line 30: | Line 30: | ||
=== Endocannabinoid Receptor 1 === | === Endocannabinoid Receptor 1 === | ||
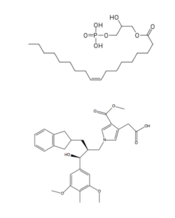
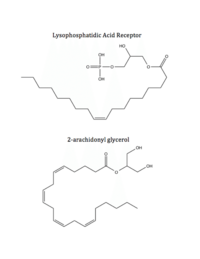
| - | LPA<sub>1</sub> is also closely related to the first of the six [http://www.nature.com/ijo/journal/v30/n1s/full/0803272a.html cannabinoid receptors]. This close relation gives CB<sub>1</sub> ([[Cannabinoid Receptor 1]]) the ability to bind to analogs of LPA and LPA<sub>1</sub> the ability to bind to analogs of CB<sub>1</sub> ligands. <ref name="regpeps">PMID: 26091040</ref> This crossing over of ligand binding opens the possibility of metabolic crosstalk between the two signaling systems. <ref name="regpeps">PMID: 26091040</ref> Complementary access to the LPA<sub>1</sub> binding pocket can be achieved by phosphorylated CB<sub>1</sub> ligand analogs, while complementary access to the CB<sub>1</sub> binding site requires dephosphorylation of LPA<sub>1</sub> ligand analogs (Figure | + | LPA<sub>1</sub> is also closely related to the first of the six [http://www.nature.com/ijo/journal/v30/n1s/full/0803272a.html cannabinoid receptors]. This close relation gives CB<sub>1</sub> ([[Cannabinoid Receptor 1]]) the ability to bind to analogs of LPA and LPA<sub>1</sub> the ability to bind to analogs of CB<sub>1</sub> ligands. <ref name="regpeps">PMID: 26091040</ref> This crossing over of ligand binding opens the possibility of metabolic crosstalk between the two signaling systems. <ref name="regpeps">PMID: 26091040</ref> Complementary access to the LPA<sub>1</sub> binding pocket can be achieved by phosphorylated CB<sub>1</sub> ligand analogs, while complementary access to the CB<sub>1</sub> binding site requires dephosphorylation of LPA<sub>1</sub> ligand analogs (Figure 5). In both cases, a ligand could serve as a primary [https://en.wikipedia.org/wiki/Selective_receptor_modulator receptor modulator] and a simultaneous [https://en.wikipedia.org/wiki/Prodrug prodrug] for a different receptor. <ref name="regpeps">PMID: 26091040</ref> |
[[Image:Ligands-1.png|200 px|left|thumb|Figure 5: Respective ligands of LPA<sub>1</sub> and CB<sub>1</sub>. LPA binds to CB<sub>1</sub> after dephosphorylation, while 2-AG binds to LPA<sub>1</sub> after phosphorylation.]] | [[Image:Ligands-1.png|200 px|left|thumb|Figure 5: Respective ligands of LPA<sub>1</sub> and CB<sub>1</sub>. LPA binds to CB<sub>1</sub> after dephosphorylation, while 2-AG binds to LPA<sub>1</sub> after phosphorylation.]] | ||
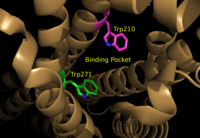
| - | <scene name='72/721543/Asp129_and_trp210/2'>Residues Asp129 and Trp210</scene> located within the hydrophobic binding pocket of LPA<sub>1</sub> may share responsibility for the preference for long unsaturated acyl chains, including the ligand LPA. The polarity of these residues provide favorable interactions between the ligand and the binding pocket.<ref name="regpeps">PMID: 26091040</ref> Additionally, Asp129 and Trp210 may serve as a trigger for agonist induced conformational changes. These residues are also interesting in regard to GPCR phylogenic evolution. <ref name="regpeps">PMID: 26091040</ref> Trp210 specifically only occurs in this position in 1% of all class A GPCR receptors and is unique to lysophospholipid and cannabinoid receptors. <ref name = 'Van Durme'>Van Durme, J., Horn, F., Costagliola, S., Vriend, G., and Vassart, G. “GRIS: glycoprotein-hormone receptor information system.” Mol. (2006) Endocrinol. 20, 2247-2255' </ref> A model for lipid agonist binding generated through molecular modeling was used to dock two of the cannabinoid receptor CB<sub>1</sub>'s most abundant endogenous ligands into the LPA<sub>1</sub> binding pocket. <ref name="regpeps">PMID: 26091040</ref> Rotameric shifts of Trp210 and Trp271 lead to the expansion of the binding pocket and the exposure of the π clouds of their indole rings. These shifts and expansion provided favorable interactions with the double bonds of the phosphorylated cannabinoid ligands (Figure | + | <scene name='72/721543/Asp129_and_trp210/2'>Residues Asp129 and Trp210</scene> located within the hydrophobic binding pocket of LPA<sub>1</sub> may share responsibility for the preference for long unsaturated acyl chains, including the ligand LPA. The polarity of these residues provide favorable interactions between the ligand and the binding pocket.<ref name="regpeps">PMID: 26091040</ref> Additionally, Asp129 and Trp210 may serve as a trigger for agonist induced conformational changes. These residues are also interesting in regard to GPCR phylogenic evolution. <ref name="regpeps">PMID: 26091040</ref> Trp210 specifically only occurs in this position in 1% of all class A GPCR receptors and is unique to lysophospholipid and cannabinoid receptors. <ref name = 'Van Durme'>Van Durme, J., Horn, F., Costagliola, S., Vriend, G., and Vassart, G. “GRIS: glycoprotein-hormone receptor information system.” Mol. (2006) Endocrinol. 20, 2247-2255' </ref> A model for lipid agonist binding generated through molecular modeling was used to dock two of the cannabinoid receptor CB<sub>1</sub>'s most abundant endogenous ligands into the LPA<sub>1</sub> binding pocket. <ref name="regpeps">PMID: 26091040</ref> Rotameric shifts of Trp210 and Trp271 lead to the expansion of the binding pocket and the exposure of the π clouds of their indole rings. These shifts and expansion provided favorable interactions with the double bonds of the phosphorylated cannabinoid ligands (Figure 6). This favorable binding provided evidence that the hydrophobic binding pockets of LPA<sub>1</sub> and CB<sub>1</sub> are able to favorably bind the same poly-unsaturated acyl chains with metabolically interconvertible head groups. <ref name="regpeps">PMID: 26091040</ref> |
[[Image:TrpRotamericShiftsCM.png|200 px|left|thumb|Figure 6: Illustration of key LPA<sub>1</sub> binding pocket residues Trp210 and Trp271. These residues provide rotameric shifts and expansion that allow for favorable interactions with phosphorylated cannabinoid ligands.]] | [[Image:TrpRotamericShiftsCM.png|200 px|left|thumb|Figure 6: Illustration of key LPA<sub>1</sub> binding pocket residues Trp210 and Trp271. These residues provide rotameric shifts and expansion that allow for favorable interactions with phosphorylated cannabinoid ligands.]] | ||
Current revision
| This Sandbox is Reserved from Jan 11 through August 12, 2016 for use in the course CH462 Central Metabolism taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1160 through Sandbox Reserved 1184. |
To get started:
More help: Help:Editing |
Lysophosphatidic Acid Receptor 1
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 Chrencik JE, Roth CB, Terakado M, Kurata H, Omi R, Kihara Y, Warshaviak D, Nakade S, Asmar-Rovira G, Mileni M, Mizuno H, Griffith MT, Rodgers C, Han GW, Velasquez J, Chun J, Stevens RC, Hanson MA. Crystal Structure of Antagonist Bound Human Lysophosphatidic Acid Receptor 1. Cell. 2015 Jun 18;161(7):1633-43. doi: 10.1016/j.cell.2015.06.002. PMID:26091040 doi:http://dx.doi.org/10.1016/j.cell.2015.06.002
- ↑ 2.0 2.1 Yung, Y. C., N. C. Stoddard, and J. Chun. "LPA Receptor Signaling: Pharmacology, Physiology, and Pathophysiology." The Journal of Lipid Research 55.7 (2014): 1192-214. Web. 17 Feb. 2016.'
- ↑ 3.0 3.1 Chun, J., Hla, T., Spiegel, S., and Moolenaar, W.H. “Lysophospholipid Receptors: Signaling and Biochemistry.” John Wiley & Sons, Inc. (2013) pp.i-xviii. 5 Feb. 2016.'
- ↑ Hernández-Méndez, Aurelio, Rocío Alcántara-Hernández, and J. Adolfo García-Sáinz. "Lysophosphatidic Acid LPA1-3 Receptors: Signaling, Regulation and in Silico Analysis of Their Putative Phosphorylation Sites." Receptors & Clinical Investigation Receptor Clin Invest 1.3 (2014). Web. 15 Feb. 2016.'
- ↑ Anliker B, Choi JW, Lin ME, Gardell SE, Rivera RR, Kennedy G, Chun J. Lysophosphatidic acid (LPA) and its receptor, LPA1 , influence embryonic schwann cell migration, myelination, and cell-to-axon segregation. Glia. 2013 Dec;61(12):2009-22. doi: 10.1002/glia.22572. Epub 2013 Sep 24. PMID:24115248 doi:http://dx.doi.org/10.1002/glia.22572
- ↑ Chun, E., Thompson, A.A., Lui, W., Roth, C.B., Griffith, M.T., Katritch, V., Kunken, J., Xu, F., Cherezov, V., Hanson, M.A., and Stevens, R.C. “Fusion partner tool chest for the stabilization and crystallization of G protein-coupled receptors.” Structure 20, (2012) 967-976.'
- ↑ Van Durme, J., Horn, F., Costagliola, S., Vriend, G., and Vassart, G. “GRIS: glycoprotein-hormone receptor information system.” Mol. (2006) Endocrinol. 20, 2247-2255'
- ↑ 8.0 8.1 8.2 8.3 Lin ME, Herr DR, Chun J. Lysophosphatidic acid (LPA) receptors: signaling properties and disease relevance. Prostaglandins Other Lipid Mediat. 2010 Apr;91(3-4):130-8. doi:, 10.1016/j.prostaglandins.2009.02.002. Epub 2009 Mar 4. PMID:20331961 doi:http://dx.doi.org/10.1016/j.prostaglandins.2009.02.002
- ↑ 9.0 9.1 9.2 Justus CR, Dong L, Yang LV. Acidic tumor microenvironment and pH-sensing G protein-coupled receptors. Front Physiol. 2013 Dec 5;4:354. doi: 10.3389/fphys.2013.00354. PMID:24367336 doi:http://dx.doi.org/10.3389/fphys.2013.00354