This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox reserved 1169
From Proteopedia
(Difference between revisions)
| Line 18: | Line 18: | ||
A major player in the transduction of the extracellular signal to the intracellular G protein is the <scene name='72/727765/Hydrogen_bonding_network/4'>hydrogen bonding network</scene> (PDB code:[http://www.rcsb.org/pdb/explore/explore.do?structureId=4GRV 4GRV)]that links the bound <font color='#32CD32'>hormone</font> with the [https://en.wikipedia.org/wiki/Hydrophobe hydrophobic] core of the <font color='#A9A9A9'>neurotensin receptor</font>. The carboxylate of L13 forms a hydrogen bond network with R327, R328, and Y324. The Tyr324, in turn, is brought into an orientation to make the formation of a <scene name='72/727765/Hydrophobic_stacking_4xee/2'>hydrophobic stacking</scene> (PDB Code:[http://www.rcsb.org/pdb/explore/explore.do?structureId=4XEE 4XEE]) network between F358, W321, A157, and F317 possible.<ref name="Krumm">PMID:23051748</ref> The effects that this network has on the activation of the intracellular G-protein was examined by the [https://en.wikipedia.org/wiki/Mutagenesis mutagenesis] of amino acids involved in this network. Mutagenesis of <scene name='72/727765/Overall_structure/12'>A86L</scene>, <scene name='72/727765/Overall_structure/13'>E166A</scene>, <scene name='72/727765/Overall_structure/14'>G125L</scene>, <scene name='72/727765/Overall_structure/15'>L310A</scene>, <scene name='72/727765/Overall_structure/16'>F358A</scene>, and <scene name='72/727765/Overall_structure/17'>V360A</scene> showed that when this interaction was disrupted, the receptor no longer was able to activate the G-protein. <ref name="Krumm"/> This discovery lead to the conclusion that the conformational changes caused by this stacking allows for the signal to be moved from the extracellular binding site through the transmembrane helices of the receptor to the intracellular region activating the G-protein. | A major player in the transduction of the extracellular signal to the intracellular G protein is the <scene name='72/727765/Hydrogen_bonding_network/4'>hydrogen bonding network</scene> (PDB code:[http://www.rcsb.org/pdb/explore/explore.do?structureId=4GRV 4GRV)]that links the bound <font color='#32CD32'>hormone</font> with the [https://en.wikipedia.org/wiki/Hydrophobe hydrophobic] core of the <font color='#A9A9A9'>neurotensin receptor</font>. The carboxylate of L13 forms a hydrogen bond network with R327, R328, and Y324. The Tyr324, in turn, is brought into an orientation to make the formation of a <scene name='72/727765/Hydrophobic_stacking_4xee/2'>hydrophobic stacking</scene> (PDB Code:[http://www.rcsb.org/pdb/explore/explore.do?structureId=4XEE 4XEE]) network between F358, W321, A157, and F317 possible.<ref name="Krumm">PMID:23051748</ref> The effects that this network has on the activation of the intracellular G-protein was examined by the [https://en.wikipedia.org/wiki/Mutagenesis mutagenesis] of amino acids involved in this network. Mutagenesis of <scene name='72/727765/Overall_structure/12'>A86L</scene>, <scene name='72/727765/Overall_structure/13'>E166A</scene>, <scene name='72/727765/Overall_structure/14'>G125L</scene>, <scene name='72/727765/Overall_structure/15'>L310A</scene>, <scene name='72/727765/Overall_structure/16'>F358A</scene>, and <scene name='72/727765/Overall_structure/17'>V360A</scene> showed that when this interaction was disrupted, the receptor no longer was able to activate the G-protein. <ref name="Krumm"/> This discovery lead to the conclusion that the conformational changes caused by this stacking allows for the signal to be moved from the extracellular binding site through the transmembrane helices of the receptor to the intracellular region activating the G-protein. | ||
== Sodium Binding Pocket == | == Sodium Binding Pocket == | ||
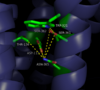
| - | Conserved across all class A GPCRs, a <scene name='72/727765/Sodium_binding_pocket_final/1'>sodium binding pocket</scene> | + | [[Image:4XEE closed sodium pocket.png|100 px|left|thumb|Figure 2: Closed form of sodium binding pocket that caps the entrance of sodium into the top of the binding pocket. PDB Code:[http://www.rcsb.org/pdb/explore/explore.do?structureId=4XEE 4XEE])]] Conserved across all class A GPCRs, a <scene name='72/727765/Sodium_binding_pocket_final/1'>sodium binding pocket</scene> PDB code:[http://www.rcsb.org/pdb/explore/explore.do?structureId=4GRV 4GRV] is seen in the middle of TM2 helix. The sodium ion is coordinated with a highly conserved Asp113 and four other oxygen contacts from a combination of water molecules. For G-protein activation to be possible, a hydrogen bond coordination with T156, S362, and N365 of the NPxxY [https://en.wikipedia.org/wiki/Structural_motif motif] must occur. Trp321 helps to maintain the active conformation of the receptor by occluding the top of the binding pocket using [https://en.wikipedia.org/wiki/Van_der_Waals_force Van der Waals] interactions (pictured top left). The form of the binding pocket where Trp321 does not occlude the top of the pocket can be seen when mutations to A86L, G215A, and V360A are present (pictured bottom left) This form of the receptor would allow more sodium into the binding pocket. The binding of sodium within this site disrupts the coordination of the hydrogen bonds and places the receptor in its uncollapsed, inactive form. <ref name="Katritch">PMID:24767681</ref> |
=== Allosteric Effects === | === Allosteric Effects === | ||
Sodium ions are a negative [https://en.wikipedia.org/wiki/Allosteric_regulation allosteric] inhibitor to the binding of the neurotensin [https://en.wikipedia.org/wiki/Agonist agonist] to the binding site on the neurotensin receptor. Sodium's binding causes for the receptor to favor its inactive state. Asp113 of the highly conserved D/RY motif and Asn365 of the highly conserved NPxxY motif form a substantial hydrogen bonding network with T156 and S362.<ref name="Krumm"/> This hydrogen bonding network prevents the incorporation of the sodium ion by collapsing upon itself and filling the sodium binding pocket. Trp321 also works to inhibit the incorporation of the sodium ion by capping off the sodium binding pocket to not allow sodium to enter from the top. Trp321 uses Van der Waals interactions to place it in the conformation necessary to block sodium from entering the site. By not allowing for sodium to enter this binding site, the receptor is able to conform to its active state and activate the G-protein that is associated with it. | Sodium ions are a negative [https://en.wikipedia.org/wiki/Allosteric_regulation allosteric] inhibitor to the binding of the neurotensin [https://en.wikipedia.org/wiki/Agonist agonist] to the binding site on the neurotensin receptor. Sodium's binding causes for the receptor to favor its inactive state. Asp113 of the highly conserved D/RY motif and Asn365 of the highly conserved NPxxY motif form a substantial hydrogen bonding network with T156 and S362.<ref name="Krumm"/> This hydrogen bonding network prevents the incorporation of the sodium ion by collapsing upon itself and filling the sodium binding pocket. Trp321 also works to inhibit the incorporation of the sodium ion by capping off the sodium binding pocket to not allow sodium to enter from the top. Trp321 uses Van der Waals interactions to place it in the conformation necessary to block sodium from entering the site. By not allowing for sodium to enter this binding site, the receptor is able to conform to its active state and activate the G-protein that is associated with it. | ||
Revision as of 06:35, 19 April 2016
Neurotensin Receptor (NTSR1)
References
- ↑ Millar RP, Newton CL. The year in G protein-coupled receptor research. Mol Endocrinol. 2010 Jan;24(1):261-74. Epub 2009 Dec 17. PMID:20019124 doi:10.1210/me.2009-0473
- ↑ Gui X, Carraway RE. Enhancement of jejunal absorption of conjugated bile acid by neurotensin in rats. Gastroenterology. 2001 Jan;120(1):151-60. PMID:11208724
- ↑ Selivonenko VG. [The interrelationship between electrolytes and phase analysis of systole in toxic goiter]. Probl Endokrinol (Mosk). 1975 Jan-Feb;21(1):19-23. PMID:1173461
- ↑ Fang Y, Lahiri J, Picard L. G protein-coupled receptor microarrays for drug discovery. Drug Discov Today. 2004 Dec 15;9(24 Suppl):S61-7. PMID:23573662
- ↑ 5.0 5.1 White JF, Noinaj N, Shibata Y, Love J, Kloss B, Xu F, Gvozdenovic-Jeremic J, Shah P, Shiloach J, Tate CG, Grisshammer R. Structure of the agonist-bound neurotensin receptor. Nature. 2012 Oct 25;490(7421):508-13. doi: 10.1038/nature11558. Epub 2012 Oct 10. PMID:23051748 doi:http://dx.doi.org/10.1038/nature11558
- ↑ Carraway R, Leeman SE. The isolation of a new hypotensive peptide, neurotensin, from bovine hypothalami. J Biol Chem. 1973 Oct 10;248(19):6854-61. PMID:4745447
- ↑ Kitabgi P. Neurotensin modulates dopamine neurotransmission at several levels along brain dopaminergic pathways. Neurochem Int. 1989;14(2):111-9. PMID:20504406
- ↑ Mustain WC, Rychahou PG, Evers BM. The role of neurotensin in physiologic and pathologic processes. Curr Opin Endocrinol Diabetes Obes. 2011 Feb;18(1):75-82. doi:, 10.1097/MED.0b013e3283419052. PMID:21124211 doi:http://dx.doi.org/10.1097/MED.0b013e3283419052
- ↑ Vincent JP, Mazella J, Kitabgi P. Neurotensin and neurotensin receptors. Trends Pharmacol Sci. 1999 Jul;20(7):302-9. PMID:10390649
- ↑ 10.0 10.1 10.2 White JF, Noinaj N, Shibata Y, Love J, Kloss B, Xu F, Gvozdenovic-Jeremic J, Shah P, Shiloach J, Tate CG, Grisshammer R. Structure of the agonist-bound neurotensin receptor. Nature. 2012 Oct 25;490(7421):508-13. doi: 10.1038/nature11558. Epub 2012 Oct 10. PMID:23051748 doi:http://dx.doi.org/10.1038/nature11558
- ↑ Katritch V, Fenalti G, Abola EE, Roth BL, Cherezov V, Stevens RC. Allosteric sodium in class A GPCR signaling. Trends Biochem Sci. 2014 May;39(5):233-44. doi: 10.1016/j.tibs.2014.03.002. Epub , 2014 Apr 21. PMID:24767681 doi:http://dx.doi.org/10.1016/j.tibs.2014.03.002
- ↑ 12.0 12.1 Valerie NC, Casarez EV, Dasilva JO, Dunlap-Brown ME, Parsons SJ, Amorino GP, Dziegielewski J. Inhibition of neurotensin receptor 1 selectively sensitizes prostate cancer to ionizing radiation. Cancer Res. 2011 Nov 1;71(21):6817-26. doi: 10.1158/0008-5472.CAN-11-1646. Epub, 2011 Sep 8. PMID:21903767 doi:http://dx.doi.org/10.1158/0008-5472.CAN-11-1646
- ↑ Kisfalvi K, Eibl G, Sinnett-Smith J, Rozengurt E. Metformin disrupts crosstalk between G protein-coupled receptor and insulin receptor signaling systems and inhibits pancreatic cancer growth. Cancer Res. 2009 Aug 15;69(16):6539-45. doi: 10.1158/0008-5472.CAN-09-0418. PMID:19679549 doi:http://dx.doi.org/10.1158/0008-5472.CAN-09-0418