We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Journal:Molecular Cell:1
From Proteopedia
(Difference between revisions)

| Line 6: | Line 6: | ||
Upon heterologous overexpression, many proteins misfold or aggregate, thus resulting in low functional yields. Human acetylcholinesterase (hAChE), an enzyme mediating synaptic transmission, is a typical case of a human protein that necessitates mammalian systems to obtain functional expression. Using a novel computational strategy, we designed an AChE variant bearing 51 mutations that improved core packing, surface polarity, and backbone rigidity. This variant expressed at ~2,000-fold higher levels in E. coli compared to wild-type hAChE, and exhibited 20°C higher thermostability with no change in enzymatic properties or in the active-site configuration as determined by crystallography. To demonstrate broad utility, we similarly designed four other human and bacterial proteins. Testing at most three designs per protein, we obtained enhanced stability and/or higher yields of soluble protein in E. coli. Our algorithm requires only a 3D structure and several dozen sequences of naturally occurring homologues, and is available at [http://pross.weizmann.ac.il| http://pross.weizmann.ac.il]. | Upon heterologous overexpression, many proteins misfold or aggregate, thus resulting in low functional yields. Human acetylcholinesterase (hAChE), an enzyme mediating synaptic transmission, is a typical case of a human protein that necessitates mammalian systems to obtain functional expression. Using a novel computational strategy, we designed an AChE variant bearing 51 mutations that improved core packing, surface polarity, and backbone rigidity. This variant expressed at ~2,000-fold higher levels in E. coli compared to wild-type hAChE, and exhibited 20°C higher thermostability with no change in enzymatic properties or in the active-site configuration as determined by crystallography. To demonstrate broad utility, we similarly designed four other human and bacterial proteins. Testing at most three designs per protein, we obtained enhanced stability and/or higher yields of soluble protein in E. coli. Our algorithm requires only a 3D structure and several dozen sequences of naturally occurring homologues, and is available at [http://pross.weizmann.ac.il| http://pross.weizmann.ac.il]. | ||
| - | <scene name='72/728277/Cv/24'>The structural underpinnings of stabilization in the designed variant dAChE4</scene>. < | + | <scene name='72/728277/Cv/24'>The structural underpinnings of stabilization in the designed variant dAChE4</scene>. <font color='blue'><b>Wild type hAChE is shown in blue</b></font> and <font color='darkorange'><b>51 mutated positions, which are distributed throughout dAChE4, are indicated by orange spheres</b></font>. |
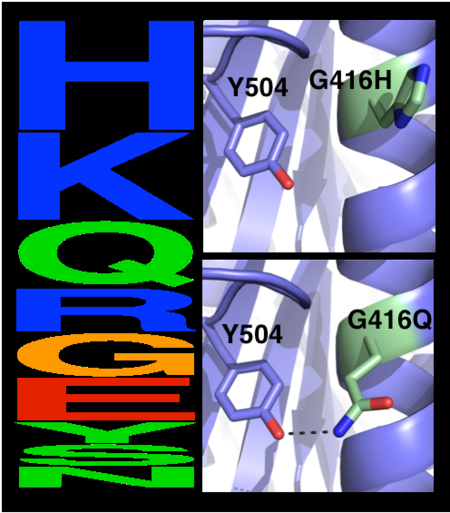
The choice of mutations at Gly416 in hAChE illustrates the role of these two filters (alignment scan and computational mutation scanning) in pruning false positives (see static image below). Position 416 is located on a partially exposed helical surface, where the small and flexible amino acid Gly is likely to destabilize hAChE. Indeed, in the alignment of 5 AChE homologues, Gly is infrequent and His is the most prevalent amino acid. Modeling shows, however, that in the specific context of hAChE, His adopts a strained side-chain conformation; in contrast, Gln, the third most prevalent amino acid, is predicted to be most stabilizing owing to its high helical propensity and favorable hydrogen-bonding with Tyr504. The combined filter therefore favors Gln over His for downstream design calculations. | The choice of mutations at Gly416 in hAChE illustrates the role of these two filters (alignment scan and computational mutation scanning) in pruning false positives (see static image below). Position 416 is located on a partially exposed helical surface, where the small and flexible amino acid Gly is likely to destabilize hAChE. Indeed, in the alignment of 5 AChE homologues, Gly is infrequent and His is the most prevalent amino acid. Modeling shows, however, that in the specific context of hAChE, His adopts a strained side-chain conformation; in contrast, Gln, the third most prevalent amino acid, is predicted to be most stabilizing owing to its high helical propensity and favorable hydrogen-bonding with Tyr504. The combined filter therefore favors Gln over His for downstream design calculations. | ||
| Line 12: | Line 12: | ||
[[Image:MC1.png|left|450px|thumb|Eliminating potentially destabilizing mutations through homologous-sequence analysis and computational mutation scanning. Left: Sequence logo for hAChE position Gly416. The height of letters represents the respective amino acid’s frequency in an alignment of homologous AChE sequences. The evolutionarily ‘allowed’ sequence space (PSSM scores ≥0) at position 416 includes the 9 amino acids shown. Right: Structural models of mutations to the evolutionarily favored amino acid His, and to Gln, which is favored by Rosetta energy calculations. The His side chain is strained due to its proximity to the bulky Tyr504 aromatic ring, whereas the Gln side chain is relaxed and forms a favorable hydrogen bond with Tyr504 (dashed line)]] | [[Image:MC1.png|left|450px|thumb|Eliminating potentially destabilizing mutations through homologous-sequence analysis and computational mutation scanning. Left: Sequence logo for hAChE position Gly416. The height of letters represents the respective amino acid’s frequency in an alignment of homologous AChE sequences. The evolutionarily ‘allowed’ sequence space (PSSM scores ≥0) at position 416 includes the 9 amino acids shown. Right: Structural models of mutations to the evolutionarily favored amino acid His, and to Gln, which is favored by Rosetta energy calculations. The His side chain is strained due to its proximity to the bulky Tyr504 aromatic ring, whereas the Gln side chain is relaxed and forms a favorable hydrogen bond with Tyr504 (dashed line)]] | ||
{{Clear}} | {{Clear}} | ||
| - | Scenes highlight stabilizing effects of <scene name='72/728277/Cv/10'>selected mutations</scene> (in <font color='red'><b>red</b></font>), <font color=' | + | Scenes highlight stabilizing effects of <scene name='72/728277/Cv/10'>selected mutations</scene> (in <font color='red'><b>red</b></font>), <font color='cyan'><b>wild type hAChE is shown in cyan</b></font> and <font color='lime'><b>designed hAChE is in green</b></font>: |
*<scene name='72/728277/Cv/4'>Buried hydrogen bonds</scene>. | *<scene name='72/728277/Cv/4'>Buried hydrogen bonds</scene>. | ||
*<scene name='72/728277/Cv/5'>Surface polarity</scene>. | *<scene name='72/728277/Cv/5'>Surface polarity</scene>. | ||
Revision as of 09:40, 5 May 2016
| |||||||||||
- ↑ REF
Proteopedia Page Contributors and Editors (what is this?)
This page complements a publication in scientific journals and is one of the Proteopedia's Interactive 3D Complement pages. For aditional details please see I3DC.