We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Aromatase
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
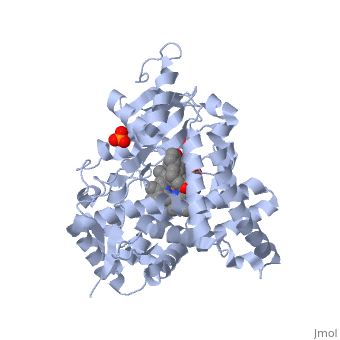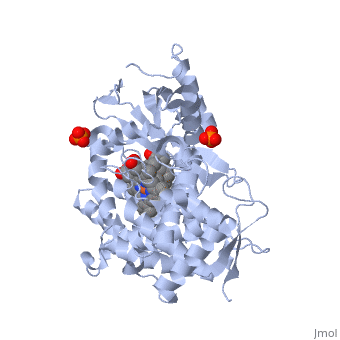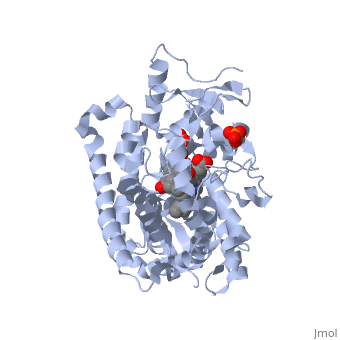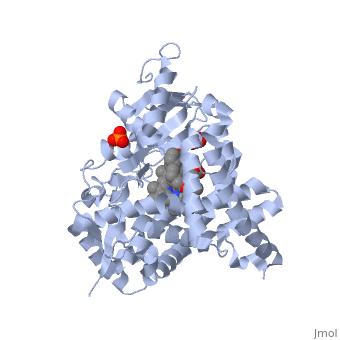
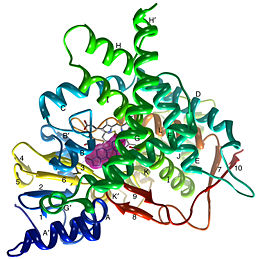
| - | < | + | <StructureSection load='3EQM' size='350' side='right' scene='' caption=''Crystal structure of human placental aromatase cytochrome P450 in complex with androstenedione [[3eqm]] '> |
| + | |||
[[Aromatase]] ([[EC]] [[Oxidoreductase|1.14.14.1]]) belongs to the [[Cytochrome P450]] family (CYP). During aromatization reactions, aromatase forms an electron-transfer complex with its partner, [[NADPH-Cytochrome_P450_Reductase|NADPH-cytochrome P450 reductase]]. This enzyme is localized in the endoplasmic reticulum of the cell, and tissue specific promoters regulate its activity.<ref> PMID:21125383 </ref> In a number of species, including humans, aromatase can be found throughout the body in places such as the brain, gonads, blood vessels, endometrium, skin, bone and tissues including the placenta and adipose tissue.<ref> PMID: 11427156 </ref> | [[Aromatase]] ([[EC]] [[Oxidoreductase|1.14.14.1]]) belongs to the [[Cytochrome P450]] family (CYP). During aromatization reactions, aromatase forms an electron-transfer complex with its partner, [[NADPH-Cytochrome_P450_Reductase|NADPH-cytochrome P450 reductase]]. This enzyme is localized in the endoplasmic reticulum of the cell, and tissue specific promoters regulate its activity.<ref> PMID:21125383 </ref> In a number of species, including humans, aromatase can be found throughout the body in places such as the brain, gonads, blood vessels, endometrium, skin, bone and tissues including the placenta and adipose tissue.<ref> PMID: 11427156 </ref> | ||
== Function == | == Function == | ||
| Line 22: | Line 23: | ||
*'''Aromatase Excess Syndrome''' | *'''Aromatase Excess Syndrome''' | ||
Research shows a rare disorder caused by excessive aromatase activity that can cause familial gynecomastia and feminization of both sexes. This can be inherited in an autosomal dominant manner, affecting females and males differently. Females with this disorder showed signs of isosexual precocity and/or macromastia. Males showed characteristics of heterosexual precocity and/or gynecomastia.<ref> PMID: 9543166 </ref> | Research shows a rare disorder caused by excessive aromatase activity that can cause familial gynecomastia and feminization of both sexes. This can be inherited in an autosomal dominant manner, affecting females and males differently. Females with this disorder showed signs of isosexual precocity and/or macromastia. Males showed characteristics of heterosexual precocity and/or gynecomastia.<ref> PMID: 9543166 </ref> | ||
| + | |||
| + | </StructureSection> | ||
==3D structures of aromatase== | ==3D structures of aromatase== | ||
Revision as of 17:51, 14 January 2017
| |||||||||||
3D structures of aromatase
See human P450 19 family in Cytochrome P450
References
- ↑ Kagawa N. Efficient expression of human aromatase (CYP19) in E. coli. Methods Mol Biol. 2011;705:109-22. PMID:21125383 doi:10.1007/978-1-61737-967-3_7
- ↑ Conley A, Hinshelwood M. Mammalian aromatases. Reproduction. 2001 May;121(5):685-95. PMID:11427156
- ↑ 3.0 3.1 3.2 3.3 3.4 Ghosh, D., Griswold, J., Erman, M., Pangborn, W. " X-ray Structure of Human Aromatase Reveals An Androgen-Specific Active Site" Journal of Steroid Biochemistry and Molecular Biology. [Online] 2010,Vol. 118, Issue 4-5, p197-202. [1]
- ↑ 4.0 4.1 "Aromatase Products" [2]
- ↑ Favia AD, Cavalli A, Masetti M, Carotti A, Recanatini M. Three-dimensional model of the human aromatase enzyme and density functional parameterization of the iron-containing protoporphyrin IX for a molecular dynamics study of heme-cysteinato cytochromes. Proteins. 2006 Mar 1;62(4):1074-87. PMID:16395678 doi:10.1002/prot.20829
- ↑ "Aromatase Inhibitors" [3]
- ↑ Jones ME, Boon WC, McInnes K, Maffei L, Carani C, Simpson ER. Recognizing rare disorders: aromatase deficiency. Nat Clin Pract Endocrinol Metab. 2007 May;3(5):414-21. PMID:17452968 doi:10.1038/ncpendmet0477
- ↑ Stratakis CA, Vottero A, Brodie A, Kirschner LS, DeAtkine D, Lu Q, Yue W, Mitsiades CS, Flor AW, Chrousos GP. The aromatase excess syndrome is associated with feminization of both sexes and autosomal dominant transmission of aberrant P450 aromatase gene transcription. J Clin Endocrinol Metab. 1998 Apr;83(4):1348-57. PMID:9543166
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, David Canner, Joel L. Sussman, Whitney Smith, Alexander Berchansky