This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Beta-2 Adrenergic Receptor
From Proteopedia
| Line 8: | Line 8: | ||
As an aside, stress during prenatal development has also been proven to impact locus coeruelus (LC) development. Nearly all adrenaline in the brain is produced and transported by neurons in the Locus Coeruleous, a small area of the brain. The locus coeruleus-Noradrenergic System (LC-NA) plays a crucial role in virtually all aspects of behavioral adaptations and performance of cognitive regions of the brain commonly affected in ASDs.<ref name="Purpura">PMID: 19059284</ref> Critical enzymes for proper LC can be downregulated by aberrant epigenetic modifications caused by prenatal stress. One well known example of this is hypomethylation of the Crh gene. When methylated, the Crh promotor is bound by the transcriptional repressor, [[MeCP2]]. Aberrant epigenetic modifications caused by prenatal stress can cause Crh promoters to be under methylated, preventing MeCp2 binding and subsequently causing overexpression of the Crh gene. Overexpression of the Crh gene is a trademark of Rett Syndrome, a well known [[Neurodevelopmental Disorders|neurodevelopmental disorder]] Interestingly, when some autism patients have a fever, the LC-NA system dysfunction abates resulting in reduced autistic behaviors. This implies that the underlying neural networks mediating the LC-NA system are still functional, and offers hope for partial reversal of ASDs through [[Pharmaceutical Drugs|pharmaceutical intervention]].<ref name="Purpura"/> | As an aside, stress during prenatal development has also been proven to impact locus coeruelus (LC) development. Nearly all adrenaline in the brain is produced and transported by neurons in the Locus Coeruleous, a small area of the brain. The locus coeruleus-Noradrenergic System (LC-NA) plays a crucial role in virtually all aspects of behavioral adaptations and performance of cognitive regions of the brain commonly affected in ASDs.<ref name="Purpura">PMID: 19059284</ref> Critical enzymes for proper LC can be downregulated by aberrant epigenetic modifications caused by prenatal stress. One well known example of this is hypomethylation of the Crh gene. When methylated, the Crh promotor is bound by the transcriptional repressor, [[MeCP2]]. Aberrant epigenetic modifications caused by prenatal stress can cause Crh promoters to be under methylated, preventing MeCp2 binding and subsequently causing overexpression of the Crh gene. Overexpression of the Crh gene is a trademark of Rett Syndrome, a well known [[Neurodevelopmental Disorders|neurodevelopmental disorder]] Interestingly, when some autism patients have a fever, the LC-NA system dysfunction abates resulting in reduced autistic behaviors. This implies that the underlying neural networks mediating the LC-NA system are still functional, and offers hope for partial reversal of ASDs through [[Pharmaceutical Drugs|pharmaceutical intervention]].<ref name="Purpura"/> | ||
| - | ==== | + | ====Structure of B2ARs==== |
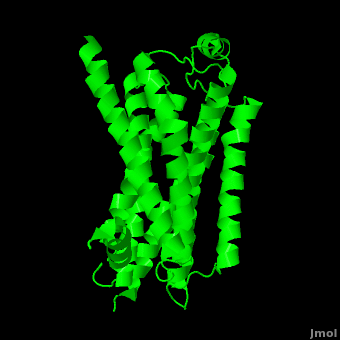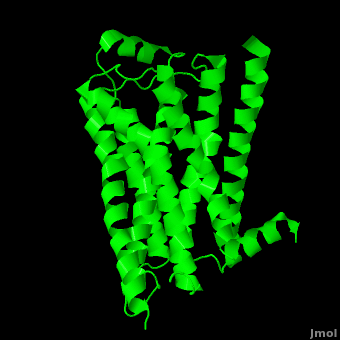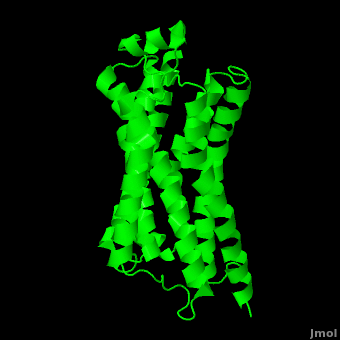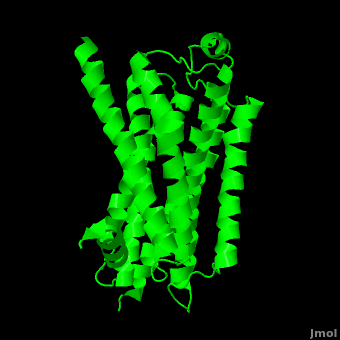
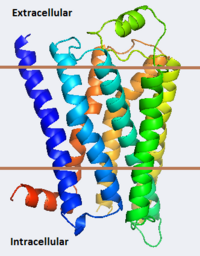
As a family, GPCRs are renowned for their structure solution difficulty. In this model, Beta2-Adrenergic Receptor (B2AR) – T4 Lysozyme fusion was developed to allow for structure solution. Fortunately, the <scene name='Beta-2_Adrenergic_Receptor/Opening_lyso/1'>lysozyme portion</scene> of the structure does not appear to impact the structure of the B2AR. The structure of B2AR is very similar to the GPCR [[Rhodopsin]], which is a photoreceptor in the retina which allows the perception of light. B2AR is a transmembrane protein with <scene name='Beta-2_Adrenergic_Receptor/Helices/1'>7 transmembrane helices</scene> and an 8th helix which runs parallel to the cytoplasmic face of the membrane.<ref name="Rasmussen">PMID: 17952055</ref> Helices 2,5,6 and 7 of B2AR have <scene name='Beta-2_Adrenergic_Receptor/Kinks/1'>kinks caused by prolines</scene> at conserved places, which are important for activation of G protein effectors. The <scene name='Beta-2_Adrenergic_Receptor/Extracellular_residues/1'>extracellular regions</scene> on all GPCRs dictate the ligand specificity of GPCRs. The partial inverse agonist, Carazolol, <scene name='Beta-2_Adrenergic_Receptor/Cara_binding/1'>binds to the receptor binding pocket</scene> of B2AR, reducing the basal activity of the receptor. This interaction involves residues Tyr 199, Ser 203, Ser 207, Phe 193, Ser 204, Ser 293, Phe 290, Tyr 308, Phe 289, Asn 312, Tyr 316, Trp 286, Trp 109 and Asp 113. Carazolol occupies a similar position as the rhodopsin inverse agonist, retinal. Carazolol does not act with the so called “toggle switch” on helix 6, but does interact with Phe 290, causing <scene name='Beta-2_Adrenergic_Receptor/Cara_binding_w/1'>Trp 286 to assume an inactive rotameric state</scene>, effectively inhibiting B2AR activity.<ref>PMID: 17962520</ref> A <scene name='Beta-2_Adrenergic_Receptor/Dry/1'>conserved DRY motif</scene> (residues 130-132) is present in all GPCRs.<ref>PMID: 18547522</ref> In rhodopsin, the Arginine in this motif forms a salt bridge with a glutamate, an interaction that maintains rhodopsin in its inactive state until it is exposed to light. In B2AR, Arg 131 <scene name='Beta-2_Adrenergic_Receptor/Dry_no_salt/1'>does not interact with Glu 268</scene>, which helps explain why B2AR has basal activity. Interestingly, Rhodopsin has no basil activity, a feature that is critical for vision.<ref>PMID: 18818650</ref> | As a family, GPCRs are renowned for their structure solution difficulty. In this model, Beta2-Adrenergic Receptor (B2AR) – T4 Lysozyme fusion was developed to allow for structure solution. Fortunately, the <scene name='Beta-2_Adrenergic_Receptor/Opening_lyso/1'>lysozyme portion</scene> of the structure does not appear to impact the structure of the B2AR. The structure of B2AR is very similar to the GPCR [[Rhodopsin]], which is a photoreceptor in the retina which allows the perception of light. B2AR is a transmembrane protein with <scene name='Beta-2_Adrenergic_Receptor/Helices/1'>7 transmembrane helices</scene> and an 8th helix which runs parallel to the cytoplasmic face of the membrane.<ref name="Rasmussen">PMID: 17952055</ref> Helices 2,5,6 and 7 of B2AR have <scene name='Beta-2_Adrenergic_Receptor/Kinks/1'>kinks caused by prolines</scene> at conserved places, which are important for activation of G protein effectors. The <scene name='Beta-2_Adrenergic_Receptor/Extracellular_residues/1'>extracellular regions</scene> on all GPCRs dictate the ligand specificity of GPCRs. The partial inverse agonist, Carazolol, <scene name='Beta-2_Adrenergic_Receptor/Cara_binding/1'>binds to the receptor binding pocket</scene> of B2AR, reducing the basal activity of the receptor. This interaction involves residues Tyr 199, Ser 203, Ser 207, Phe 193, Ser 204, Ser 293, Phe 290, Tyr 308, Phe 289, Asn 312, Tyr 316, Trp 286, Trp 109 and Asp 113. Carazolol occupies a similar position as the rhodopsin inverse agonist, retinal. Carazolol does not act with the so called “toggle switch” on helix 6, but does interact with Phe 290, causing <scene name='Beta-2_Adrenergic_Receptor/Cara_binding_w/1'>Trp 286 to assume an inactive rotameric state</scene>, effectively inhibiting B2AR activity.<ref>PMID: 17962520</ref> A <scene name='Beta-2_Adrenergic_Receptor/Dry/1'>conserved DRY motif</scene> (residues 130-132) is present in all GPCRs.<ref>PMID: 18547522</ref> In rhodopsin, the Arginine in this motif forms a salt bridge with a glutamate, an interaction that maintains rhodopsin in its inactive state until it is exposed to light. In B2AR, Arg 131 <scene name='Beta-2_Adrenergic_Receptor/Dry_no_salt/1'>does not interact with Glu 268</scene>, which helps explain why B2AR has basal activity. Interestingly, Rhodopsin has no basil activity, a feature that is critical for vision.<ref>PMID: 18818650</ref> | ||
Revision as of 18:39, 12 July 2017
| |||||||||||
Contents |
3D structures of beta-2 adrenergic receptor
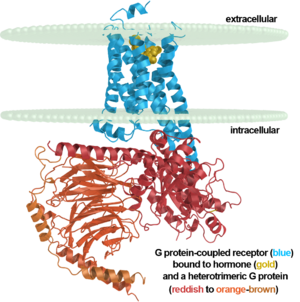
β2 adrenergic receptor binding a hormone analog |
| |||||||||||
| |||||||||||
Nobel Prize Related to the Structures
Robert J. Lefkowitz and Brian K. Kobilka share the 2012 Nobel Prize in Chemistry for work on GPCRs that includes solving the first structures of a ligand-activated GPCR (2r4r, 2r4s, & 2rh1 in 2007)[1][2][3] and the first activated GPCR in complex with its G protein (3sn6 in 2011)[4][5][6][7]. A detailed description of the laureates' body of work on this class of receptors with images is here.
References
- ↑ 1.0 1.1 1.2 Witter FR, Zimmerman AW, Reichmann JP, Connors SL. In utero beta 2 adrenergic agonist exposure and adverse neurophysiologic and behavioral outcomes. Am J Obstet Gynecol. 2009 Dec;201(6):553-9. PMID:19961985 doi:10.1016/j.ajog.2009.07.010
- ↑ 2.0 2.1 Mehler MF, Purpura DP. Autism, fever, epigenetics and the locus coeruleus. Brain Res Rev. 2009 Mar;59(2):388-92. Epub 2008 Nov 24. PMID:19059284 doi:10.1016/j.brainresrev.2008.11.001
- ↑ Rasmussen SG, Choi HJ, Rosenbaum DM, Kobilka TS, Thian FS, Edwards PC, Burghammer M, Ratnala VR, Sanishvili R, Fischetti RF, Schertler GF, Weis WI, Kobilka BK. Crystal structure of the human beta2 adrenergic G-protein-coupled receptor. Nature. 2007 Nov 15;450(7168):383-7. Epub 2007 Oct 21. PMID:17952055 doi:10.1038/nature06325
- ↑ Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Kuhn P, Weis WI, Kobilka BK, Stevens RC. High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science. 2007 Nov 23;318(5854):1258-65. Epub 2007 Oct 25. PMID:17962520
- ↑ Hanson MA, Cherezov V, Griffith MT, Roth CB, Jaakola VP, Chien EY, Velasquez J, Kuhn P, Stevens RC. A specific cholesterol binding site is established by the 2.8 A structure of the human beta2-adrenergic receptor. Structure. 2008 Jun;16(6):897-905. PMID:18547522 doi:10.1016/j.str.2008.05.001
- ↑ Scheerer P, Park JH, Hildebrand PW, Kim YJ, Krauss N, Choe HW, Hofmann KP, Ernst OP. Crystal structure of opsin in its G-protein-interacting conformation. Nature. 2008 Sep 25;455(7212):497-502. PMID:18818650 doi:10.1038/nature07330
- ↑ Warne T, Moukhametzianov R, Baker JG, Nehme R, Edwards PC, Leslie AG, Schertler GF, Tate CG. The structural basis for agonist and partial agonist action on a beta(1)-adrenergic receptor. Nature. 2011 Jan 13;469(7329):241-4. PMID:21228877 doi:10.1038/nature09746
- ↑ Cruickshank JM. Beta blockers in hypertension. Lancet. 2010 Aug 7;376(9739):415; author reply 415-6. PMID:20692524 doi:10.1016/S0140-6736(10)61217-2
See Also
- G protein-coupled receptor
- Adrenergic receptor
- The Madison West High School 2008 SMART Team's Page on the β-2 adrenergic receptor
- Nobel Prizes for 3D Molecular Structure
- Highest impact structures of all time
- G proteins
- Rhodopsin
- GTP-binding protein
- Pharmaceutical Drugs
- Membrane proteins
- Hormone
External Resources
- Robert J. Lefkowitz and Brian K. Kobilka share the 2012 Nobel Prize in Chemistry for work on GPCRs that includes solving the first structures of a ligand-activated GPCR (2007) and the first activated GPCR in complex with its G protein (2011). A detailed description of the laureates' body of work on this class of receptors with images is here.
- The April 2008 RCSB PDB Molecule of the Month feature on Adrenergic Receptors by David S. Goodsell is 10.2210/rcsb_pdb/mom_2008_4.
Page Development
This article was initially developed based on lectures given in Chemistry 543 by Prof. Clarence E. Schutt at Princeton University.
Proteopedia Page Contributors and Editors (what is this?)
Wayne Decatur, David Canner, Alexander Berchansky, Dotan Shaniv, Joel L. Sussman, Michal Harel