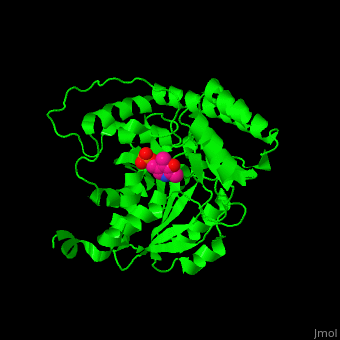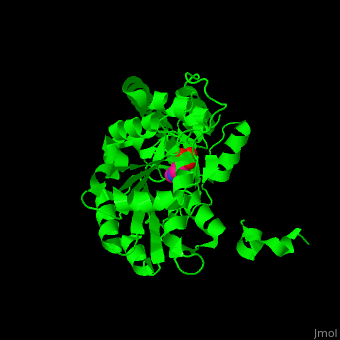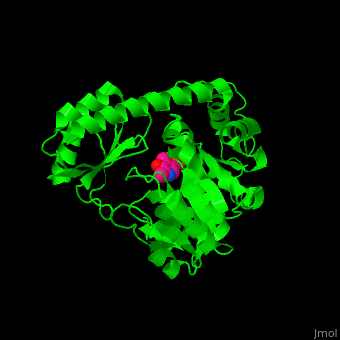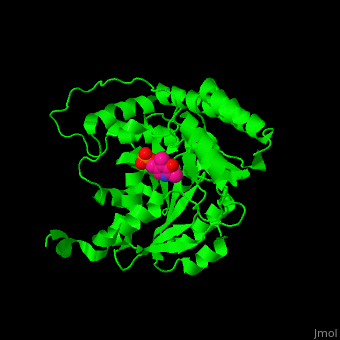
Aminotransferase
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | <StructureSection load=' | + | <StructureSection load='' size='350' side='right' caption='E. coli Histidinol-phosphate aminotransferase complex with PLP (PDB code [[1gew]])' scene='72/721044/Cv/1'> |
__TOC__ | __TOC__ | ||
== Function == | == Function == | ||
Revision as of 19:44, 18 October 2017
| |||||||||||
3D structures of aminotransferase
Updated on 18-October-2017
References
- ↑ Mizuguchi H, Hayashi H, Miyahara I, Hirotsu K, Kagamiyama H. Characterization of histidinol phosphate aminotransferase from Escherichia coli. Biochim Biophys Acta. 2003 Apr 11;1647(1-2):321-4. PMID:12686152
- ↑ Kirsch JF, Eichele G, Ford GC, Vincent MG, Jansonius JN, Gehring H, Christen P. Mechanism of action of aspartate aminotransferase proposed on the basis of its spatial structure. J Mol Biol. 1984 Apr 15;174(3):497-525. PMID:6143829 doi:http://dx.doi.org/10.1016/0022-2836(84)90333-4
- ↑ Kirsch JF, Eichele G, Ford GC, Vincent MG, Jansonius JN, Gehring H, Christen P. Mechanism of action of aspartate aminotransferase proposed on the basis of its spatial structure. J Mol Biol. 1984 Apr 15;174(3):497-525. PMID:6143829 doi:http://dx.doi.org/10.1016/0022-2836(84)90333-4
- ↑ Haruyama K, Nakai T, Miyahara I, Hirotsu K, Mizuguchi H, Hayashi H, Kagamiyama H. Structures of Escherichia coli histidinol-phosphate aminotransferase and its complexes with histidinol-phosphate and N-(5'-phosphopyridoxyl)-L-glutamate: double substrate recognition of the enzyme. Biochemistry. 2001 Apr 17;40(15):4633-44. PMID:11294630
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Joel L. Sussman, Jaime Prilusky