We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox k11v
From Proteopedia
(Difference between revisions)
| Line 12: | Line 12: | ||
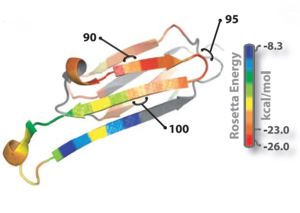
[[Image:Rosetta_image.jpg | thumb | 300px | right | Fig. 1 : Ribbon diagram of a single subunit of ABC (16), colored by propensity to form amyloid, with red being the highest and blue the lowest propensity. The segment from residue 90 to 100, termed K11V, forms the cylindrin. ]] | [[Image:Rosetta_image.jpg | thumb | 300px | right | Fig. 1 : Ribbon diagram of a single subunit of ABC (16), colored by propensity to form amyloid, with red being the highest and blue the lowest propensity. The segment from residue 90 to 100, termed K11V, forms the cylindrin. ]] | ||
| - | The entire 11-residue segment KVKVLGDVIEV forms a <scene name='77/771966/K11v_in_black/ | + | The entire 11-residue segment KVKVLGDVIEV forms a <scene name='77/771966/K11v_in_black/2'>hairpin loop</scene> in the 3D structure of ABC.The six residue segment GDVIEV ,termed <scene name='77/771966/Gdviev_black/1'>G6V</scene>,forms fibrils and microcrystals.The microcrystals enabled us to determine the atomic structure of G6V, which proved to be a standard class 2 steric zipper, essentially an amyloid-like protofilament. |
Amyloid fibrils and oligomer are both formed by the hairpin segment KVKVLGDVIEV.K11V forms fibrils similar to those of protein(ABC) on shaking at elevated temperature and also similar to K11V^V2L(K11V-TR).The fibrils diameter range from 20 to 100 nm in electrom microscope.G6V,K11V,K11V-TR are all convertible to amyloid state,as is their parent protein ABC.Segment K11V,K11V-TR,and a sequence variant with Leu replacing Val at position 2(K11V^2L) forms stable oligomers intermediate in size between monomer and fiber. | Amyloid fibrils and oligomer are both formed by the hairpin segment KVKVLGDVIEV.K11V forms fibrils similar to those of protein(ABC) on shaking at elevated temperature and also similar to K11V^V2L(K11V-TR).The fibrils diameter range from 20 to 100 nm in electrom microscope.G6V,K11V,K11V-TR are all convertible to amyloid state,as is their parent protein ABC.Segment K11V,K11V-TR,and a sequence variant with Leu replacing Val at position 2(K11V^2L) forms stable oligomers intermediate in size between monomer and fiber. | ||
Revision as of 14:11, 10 November 2017
Toxic Amyloid Small Oligomer’s atomic view
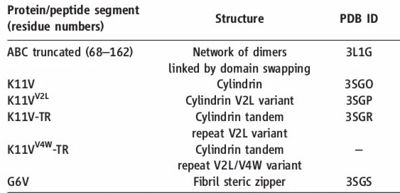
| |||||||||||
References
- ↑ Laganowsky A, Liu C, Sawaya MR, Whitelegge JP, Park J, Zhao M, Pensalfini A, Soriaga AB, Landau M, Teng PK, Cascio D, Glabe C, Eisenberg D. Atomic view of a toxic amyloid small oligomer. Science. 2012 Mar 9;335(6073):1228-31. PMID:22403391 doi:10.1126/science.1213151