Aspartate carbamoyltransferase
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
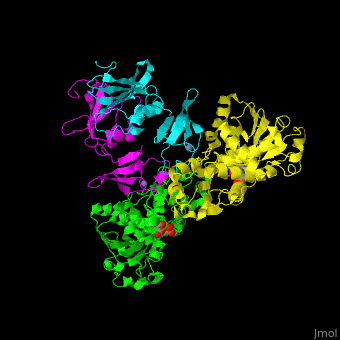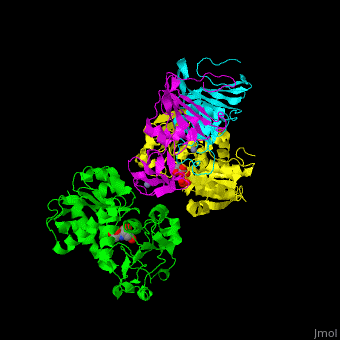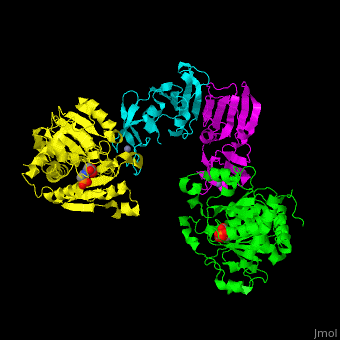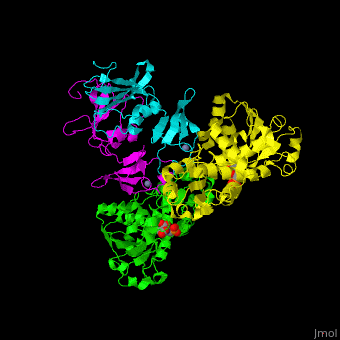
| - | <StructureSection load=' | + | <StructureSection load='' size='350' side='right' caption='Structure of E. coli aspartate carbamoyltransferase catalytic (grey and pink) and regulatory (green and yellow) subunits complex with inhibitor PALA and Zn+2 ions (grey) (PDB code [[1d09]]).' scene='59/592660/Cv/2'> |
__TOC__ | __TOC__ | ||
== Function == | == Function == | ||
| Line 23: | Line 23: | ||
**[[2qg9]], [[2qgf]] – EcATC C + R (mutant) subunits <br /> | **[[2qg9]], [[2qgf]] – EcATC C + R (mutant) subunits <br /> | ||
**[[3csu]] – EcATC C <br /> | **[[3csu]] – EcATC C <br /> | ||
| - | **[[ | + | **[[5vmq]], [[3npm]] – EcATC C (mutant) <br /> |
**[[2be7]] – ATC C (mutant) + R subunits – ''Moritella profunda'' <br /> | **[[2be7]] – ATC C (mutant) + R subunits – ''Moritella profunda'' <br /> | ||
**[[2at2]], [[3r7d]], [[3r7f]] – BsATC – ''Bacillus subtilis''<br /> | **[[2at2]], [[3r7d]], [[3r7f]] – BsATC – ''Bacillus subtilis''<br /> | ||
Revision as of 20:12, 28 December 2017
| |||||||||||
3D structures of aspartate carbamoyltransferase
Updated on 28-December-2017
References
- ↑ Jin L, Stec B, Lipscomb WN, Kantrowitz ER. Insights into the mechanisms of catalysis and heterotropic regulation of Escherichia coli aspartate transcarbamoylase based upon a structure of the enzyme complexed with the bisubstrate analogue N-phosphonacetyl-L-aspartate at 2.1 A. Proteins. 1999 Dec 1;37(4):729-42. PMID:10651286