We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Epidermal Growth Factor Receptor
From Proteopedia
(Difference between revisions)
| Line 21: | Line 21: | ||
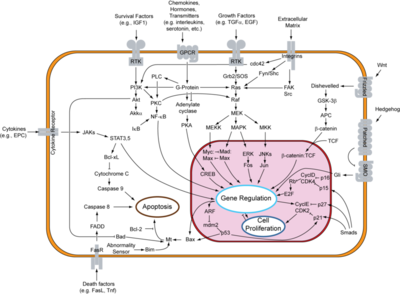
[[Gefitinib]] is an inhibitor of EGFR, and it is used to treat some cancers especially non-small cell lung cancers (NSCLC) <ref name='pao'/>. If one is able to inhibit the ability of EGF to bind to its receptor, one can control the rate of cell division in tumor cells caused by mutations or problems in EGFR. Gefitinib binds to EGFR at its highly important ATP binding activation site which is located at lysine 745 <ref name='pao'/>. When Gefitinib binds to this ATP binding site, it obviously does not allow for ATP to attach and phosphorylate in order to activate EGFR (<scene name='Yousuf_Bahrami_sandbox_1/Egfr_binded_to_gefitinib/1'>Gefitinib binded to EGFR</scene>). If ATP cannot bind, less EGF receptors will be activated and therefore this should help inhibit cell division in tumor cells and even cause regression. According to a study with two phase trials, regression of tumors were found in 28% of patients treated in Japan and 10% of those treated in Europe and U.S.A. <ref name='pao'/>. | [[Gefitinib]] is an inhibitor of EGFR, and it is used to treat some cancers especially non-small cell lung cancers (NSCLC) <ref name='pao'/>. If one is able to inhibit the ability of EGF to bind to its receptor, one can control the rate of cell division in tumor cells caused by mutations or problems in EGFR. Gefitinib binds to EGFR at its highly important ATP binding activation site which is located at lysine 745 <ref name='pao'/>. When Gefitinib binds to this ATP binding site, it obviously does not allow for ATP to attach and phosphorylate in order to activate EGFR (<scene name='Yousuf_Bahrami_sandbox_1/Egfr_binded_to_gefitinib/1'>Gefitinib binded to EGFR</scene>). If ATP cannot bind, less EGF receptors will be activated and therefore this should help inhibit cell division in tumor cells and even cause regression. According to a study with two phase trials, regression of tumors were found in 28% of patients treated in Japan and 10% of those treated in Europe and U.S.A. <ref name='pao'/>. | ||
| - | The real question to answer was why is gefitinib effective against some tumors and not against others. Through studying ten patients who showed response to gefitinib, 70% had mutations in exons 18-24 of EGFR using mutational profiling of exons 18-24 <ref name='pao'/>. Of these 7 patients, six showed the exact same mutation at the critical ATP binding site of K745 (<scene name='Yousuf_Bahrami_sandbox_1/K745/1'>See K745</scene>) while the other patient showed a L858R mutation (<scene name='Yousuf_Bahrami_sandbox_1/858/1'>See L858</scene>) <ref name='pao'/>. These two mutations seem to be the two prevalent mutations seen in patients that have shown responses to | + | The real question to answer was why is gefitinib effective against some tumors and not against others. Through studying ten patients who showed response to gefitinib, 70% had mutations in exons 18-24 of EGFR using mutational profiling of exons 18-24 <ref name='pao'/>. Of these 7 patients, six showed the exact same mutation at the critical ATP binding site of K745 (<scene name='Yousuf_Bahrami_sandbox_1/K745/1'>See K745</scene>) while the other patient showed a L858R mutation (<scene name='Yousuf_Bahrami_sandbox_1/858/1'>See L858</scene>) <ref name='pao'/>. These two mutations seem to be the two prevalent mutations seen in patients that have shown responses to [[Gefitinib]]. Through studying 8 refractory tumors not affected by gefitinib, none of the eight showed any mutations in exons 18-24 of EGFR <ref name='pao'/>. This shows the connection between a mutation in EGFR and its ability to be treated by gefitinib which binds to the critical ATP binding site. Many of these mutations occurred at the same ATP binding site. Another drug, [[Erlotinib]], showed almost exactly the same results as gefitinib <ref name='pao'/>. |
Ten percent of lung cancers occur in patients who are deemed to be "never smokers" which are people who have smoked less than 100 cigarettes in lifetime <ref name='pao'/>. Therefore, a large number of people are affected by lung cancer without smoking, and this is quite important. What do these people have in common if they are not smoking cigarettes? A study showed that 75% of cancers with a mutation in EGFR were from these "never smokers" <ref name='pao'/>. This means that gefitinib and erlotinib are most likely going to be effective on people who have not smoked because there is a high correlation of them having these mutations in EGFR. Studying these mutations and what causes them could be the next step in understanding how to prevent these types of lung cancers. | Ten percent of lung cancers occur in patients who are deemed to be "never smokers" which are people who have smoked less than 100 cigarettes in lifetime <ref name='pao'/>. Therefore, a large number of people are affected by lung cancer without smoking, and this is quite important. What do these people have in common if they are not smoking cigarettes? A study showed that 75% of cancers with a mutation in EGFR were from these "never smokers" <ref name='pao'/>. This means that gefitinib and erlotinib are most likely going to be effective on people who have not smoked because there is a high correlation of them having these mutations in EGFR. Studying these mutations and what causes them could be the next step in understanding how to prevent these types of lung cancers. | ||
Revision as of 09:57, 2 January 2018
| |||||||||||
3D Structures of Epidermal Growth Factor Receptor
Updated on 02-January-2018
Additional Resources
For additional information, see: Cancer
References
- ↑ D'Uva G, Aharonov A, Lauriola M, Kain D, Yahalom-Ronen Y, Carvalho S, Weisinger K, Bassat E, Rajchman D, Yifa O, Lysenko M, Konfino T, Hegesh J, Brenner O, Neeman M, Yarden Y, Leor J, Sarig R, Harvey RP, Tzahor E. ERBB2 triggers mammalian heart regeneration by promoting cardiomyocyte dedifferentiation and proliferation. Nat Cell Biol. 2015 May;17(5):627-38. doi: 10.1038/ncb3149. Epub 2015 Apr 6. PMID:25848746 doi:http://dx.doi.org/10.1038/ncb3149
- ↑ 2.0 2.1 Sherrill JM, Kyte J. Activation of epidermal growth factor receptor by epidermal growth factor. Biochemistry. 1996 May 7;35(18):5705-18. doi: 10.1021/bi9602268. PMID:8639530 doi:http://dx.doi.org/10.1021/bi9602268
- ↑ 3.0 3.1 Herbst RS. Review of epidermal growth factor receptor biology. Int J Radiat Oncol Biol Phys. 2004;59(2 Suppl):21-6. doi:, 10.1016/j.ijrobp.2003.11.041. PMID:15142631 doi:http://dx.doi.org/10.1016/j.ijrobp.2003.11.041
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, Singh B, Heelan R, Rusch V, Fulton L, Mardis E, Kupfer D, Wilson R, Kris M, Varmus H. EGF receptor gene mutations are common in lung cancers from "never smokers" and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004 Sep 7;101(36):13306-11. doi:, 10.1073/pnas.0405220101. Epub 2004 Aug 25. PMID:15329413 doi:http://dx.doi.org/10.1073/pnas.0405220101
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, David Canner, Alexander Berchansky, Joel L. Sussman, Jaime Prilusky