Beta-2 Adrenergic Receptor
From Proteopedia
| Line 1: | Line 1: | ||
| - | <StructureSection load=' | + | <StructureSection load='' size='490' side='right' caption='Solved Structure of a Beta 2-Adrenergic Receptor, ([[2rh1]])' scene='Beta-2_Adrenergic_Receptor/Opening/1' > |
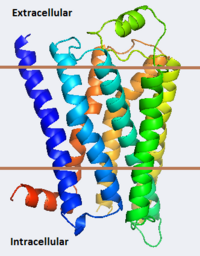
[[Image:B2ar Image3.png|200px|left]] [[Beta-2 Adrenergic Receptor]]'''s''' (B2ARs) are a type of [[G protein-coupled receptor|G Protein-Coupled Receptor (GPCR)]]. GPCRs are the largest family of integral membrane proteins in the human body with over 1000 unique Isoforms. B2AR is activated by hormone ligands like adrenaline (epinephrine) and noradrenaline and plays a critical role in cardiovascular and pulmonary physiology. Binding of adrenaline by B2AR causes a sympathetic nervous system response like the well-known “fight or flight response”, resulting in an increased heart rate, pupil dilation, rapid energy mobilization and diversion of blood to skeletal muscle. More precisely, upon binding a ligand, B2AR activates [[Adenylyl cyclase]] through interaction with B2ARs C-terminus. Adenylyl cyclase subsequently converts ATP into cAMP, which functions as a downstream signaling molecule activating effectors like cAMP-dependent protein kinases, resulting in various bodily responses.<ref name="Witter"/> | [[Image:B2ar Image3.png|200px|left]] [[Beta-2 Adrenergic Receptor]]'''s''' (B2ARs) are a type of [[G protein-coupled receptor|G Protein-Coupled Receptor (GPCR)]]. GPCRs are the largest family of integral membrane proteins in the human body with over 1000 unique Isoforms. B2AR is activated by hormone ligands like adrenaline (epinephrine) and noradrenaline and plays a critical role in cardiovascular and pulmonary physiology. Binding of adrenaline by B2AR causes a sympathetic nervous system response like the well-known “fight or flight response”, resulting in an increased heart rate, pupil dilation, rapid energy mobilization and diversion of blood to skeletal muscle. More precisely, upon binding a ligand, B2AR activates [[Adenylyl cyclase]] through interaction with B2ARs C-terminus. Adenylyl cyclase subsequently converts ATP into cAMP, which functions as a downstream signaling molecule activating effectors like cAMP-dependent protein kinases, resulting in various bodily responses.<ref name="Witter"/> | ||
Revision as of 19:42, 6 January 2018
| |||||||||||
Contents |
3D structures of beta-2 adrenergic receptor
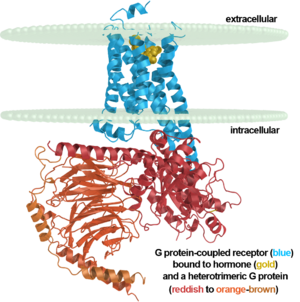
β2 adrenergic receptor binding a hormone analog |
| |||||||||||
| |||||||||||
Nobel Prize Related to the Structures
Robert J. Lefkowitz and Brian K. Kobilka share the 2012 Nobel Prize in Chemistry for work on GPCRs that includes solving the first structures of a ligand-activated GPCR (2r4r, 2r4s, & 2rh1 in 2007)[1][2][3] and the first activated GPCR in complex with its G protein (3sn6 in 2011)[4][5][6][7]. A detailed description of the laureates' body of work on this class of receptors with images is here.
References
- ↑ 1.0 1.1 1.2 Witter FR, Zimmerman AW, Reichmann JP, Connors SL. In utero beta 2 adrenergic agonist exposure and adverse neurophysiologic and behavioral outcomes. Am J Obstet Gynecol. 2009 Dec;201(6):553-9. PMID:19961985 doi:10.1016/j.ajog.2009.07.010
- ↑ 2.0 2.1 Mehler MF, Purpura DP. Autism, fever, epigenetics and the locus coeruleus. Brain Res Rev. 2009 Mar;59(2):388-92. Epub 2008 Nov 24. PMID:19059284 doi:10.1016/j.brainresrev.2008.11.001
- ↑ Rasmussen SG, Choi HJ, Rosenbaum DM, Kobilka TS, Thian FS, Edwards PC, Burghammer M, Ratnala VR, Sanishvili R, Fischetti RF, Schertler GF, Weis WI, Kobilka BK. Crystal structure of the human beta2 adrenergic G-protein-coupled receptor. Nature. 2007 Nov 15;450(7168):383-7. Epub 2007 Oct 21. PMID:17952055 doi:10.1038/nature06325
- ↑ Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Kuhn P, Weis WI, Kobilka BK, Stevens RC. High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science. 2007 Nov 23;318(5854):1258-65. Epub 2007 Oct 25. PMID:17962520
- ↑ Hanson MA, Cherezov V, Griffith MT, Roth CB, Jaakola VP, Chien EY, Velasquez J, Kuhn P, Stevens RC. A specific cholesterol binding site is established by the 2.8 A structure of the human beta2-adrenergic receptor. Structure. 2008 Jun;16(6):897-905. PMID:18547522 doi:10.1016/j.str.2008.05.001
- ↑ Scheerer P, Park JH, Hildebrand PW, Kim YJ, Krauss N, Choe HW, Hofmann KP, Ernst OP. Crystal structure of opsin in its G-protein-interacting conformation. Nature. 2008 Sep 25;455(7212):497-502. PMID:18818650 doi:10.1038/nature07330
- ↑ Warne T, Moukhametzianov R, Baker JG, Nehme R, Edwards PC, Leslie AG, Schertler GF, Tate CG. The structural basis for agonist and partial agonist action on a beta(1)-adrenergic receptor. Nature. 2011 Jan 13;469(7329):241-4. PMID:21228877 doi:10.1038/nature09746
- ↑ Cruickshank JM. Beta blockers in hypertension. Lancet. 2010 Aug 7;376(9739):415; author reply 415-6. PMID:20692524 doi:10.1016/S0140-6736(10)61217-2
See Also
- G protein-coupled receptor
- Adrenergic receptor
- The Madison West High School 2008 SMART Team's Page on the β-2 adrenergic receptor
- Nobel Prizes for 3D Molecular Structure
- Highest impact structures of all time
- G proteins
- Rhodopsin
- GTP-binding protein
- Pharmaceutical Drugs
- Membrane proteins
- Hormone
External Resources
- Robert J. Lefkowitz and Brian K. Kobilka share the 2012 Nobel Prize in Chemistry for work on GPCRs that includes solving the first structures of a ligand-activated GPCR (2007) and the first activated GPCR in complex with its G protein (2011). A detailed description of the laureates' body of work on this class of receptors with images is here.
- The April 2008 RCSB PDB Molecule of the Month feature on Adrenergic Receptors by David S. Goodsell is 10.2210/rcsb_pdb/mom_2008_4.
Page Development
This article was initially developed based on lectures given in Chemistry 543 by Prof. Clarence E. Schutt at Princeton University.
Proteopedia Page Contributors and Editors (what is this?)
Wayne Decatur, David Canner, Alexander Berchansky, Dotan Shaniv, Joel L. Sussman, Michal Harel