|
Function
Polyadenylation Complex
Hrp1 is a member of yeast mRNA cleavage factor 1 (CF1), which, along with the cleavage stimulatory factor (CstF), processes the 3’ end, most notably through polyadenylation. Though crucial in eukaryotic pre-mRNA processing, polyadenylation is especially important in yeast, where intron splicing is far less frequent than in higher eukaryotes.[3] CF1 binds mRNA upstream of the cleavage site[4] and is divided into two components, CF1a and b, the former of which contains Rna14, Rna15, Clp1 and Pfc11.[5] Hrp1 is the sole polypeptide of cleavage factor 1b (CF1b).[6] Hrp1 interacts with the CF1a proteins Rna14 and Rna15 in an inverted U-like structure in the presence of mRNA.[7] Rna15 interacts with both RNA recognition motifs (RRMs) of Hrp15[8], while Rna14 contacts Hrp1 in such a way that maximizes the distance between the negative domains of each protein.[9]
Specificity and Location
Yeast hnRNPs, including Hrp1, are specific to certain mRNA strands. Hrp1 is specific to an mRNA efficiency element consisting of alternating UA sequencing[10] approximately seven nucleotides in length.[11] This sequence specificity, among other observational data, has given credence to the notion, first proposed by Minvielle-Sebastia, et al., that Hrp1 is not totally essential to the 3’ processing of every mRNA.[12] This notion is further supported by the ability of yeast cells to survive hyperosmotic stress-induced extranuclear export of Hrp1.[13]
Hrp1 participates in mRNA processing within the nucleus, but it may be found endo- or exonuclearly.[14],[15] A nuclear localization signal (NLS) at the C-terminal end of Hrp1 is essential to its recognition by the nuclear transportin receptor Kap104. The receptor and NLS are orthologous to human karyopherin B2 and hnRNP A1.[16]
Regulatory Function
Hrp1 participates in multiple regulatory pathways involving 3’ processing.[17] It has been implicated in the inhibition of transcription by the Sen-1 helicase as mediated by Nrd-1.[18] Hrp1 also activates the nonsense-mediated mRNA decay pathway, which monitors translation and degrades incorrect mRNA.[19] Hrp1, along with Rna14, competes with Npl13, which inhibits cleavage at the polyadenylation site.[20]
Structure
HRP1 is made up of two RNA binding domains (RBDs) that contain residues serving to facilitate RNA recognition. These two domains fold into a βαββαβ secondary structure[21] in an RNA-free environment, allowing Hrp1 to behave rigidly. The includes residues extending from Ser158 to Ala233 and the extends from Lys244 to Ala318. Both RBDs are composed of a four-stranded beta sheet with two alpha helices spanning across one side of the sheet. The linker region is made up of residues spanning from Ile234 to Gly243. When RNA is introduced into the environment, conformational change is demonstrated within the linker region and a forms from Arg236 to Lys241. The helix that forms is made up of many charged polar residues that stablilize themselves through between Arg236-Asp240 and Asp237-Lys241.[22]
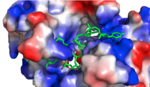 Figure 1:Positively charged cleft within HRP1 in which RNA binds Hrp1 identifies the polyadenylation enhancement element (PEE) in the 3' UTR of RNA through Van Der Waals interactions and hydrogen bonding. The PEE is made up of UAUAUA sequence in which HRP1 recognizes and binds. The association of these RBD domains aids in binding through the development of a deep highly positively charged cleft between the two. This cleft is v-shaped, accommodating the RNA very well. Some RNA bases have been revealed to be buried within protein pockets while some are observed in between protein loops. The recognition of RNA by Hrp1 is entirely dominated by RNA-protein interactions. The absence of RNA base pairing or RNA base-base stacking which have been observed in similar complexes might explain the ability of Hrp1 to target short RNA sequences in comparison to those recognized by similar proteins.
RNA binding
Recognition Specificity
HRP1 is able to discriminate RNA from DNA due to ribose-specific hydrogen bonds, such as the ones found between the 2’OH of Ade2 and phosphate oxygen of Ura3, and between the 2’OH of Ade6 and 5’O of Ura7. Furthermore, the residues found in the binding site of HRP1 have specific interactions with each individual base of the 5’-AUAUAU’3’ RNA sequence. The adenines are deeply buried in hydrophobic pockets, and uracil recognition is mainly dependent on Van der Waals interactions.
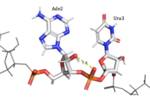 Figure 2:Ade2 donating a hydrogen to the phosphate O of Ura3. 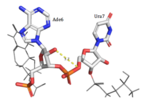 Figure 3:Ade6 donating a hydrogen to the 5'O of Ura7. Adenosine recognition
HRP1 specifically binds 3 Adenosine ribonucleotides within the PEE. Adenosine recognition is facilitated through the use of hydrophobic pockets found within HRP1. binding is made possible through the interaction of Phe246, which makes up the foundation of the recognition pocket. In addition, the C-terminal of Arg321 interacts with the opposite side of Ade2 through pi-cation interactions. Upon binding, is fit inside of a deep hydrophobic pocket made up of Trp168 and Lys226. The stacking of Trp in this interaction is demonstrated as a unique feature of Hrp1; similar Hrp1-like proteins maintain this conserved Trp, but do not demonstrate Trp stacking. The hydrophobic pocket in which resides upon binding is made up of Phe162 and Ile234, which sandwich Ade6. In addition, the three Adenosines participating in binding are also recognized by Hydrogen bonds to bases that determine specificity. The three Adenosines recognized display 1, 3, and 2 Hydrogen Bond(s), respectively. The three backbone amides (Glu319 NH, Trp168 NH, Ile234 NH) hydrogen bond with nitrogen atoms of the three adenosine bases (Ade2 N1, Ade4 N7, Ade6 N1). In addition, Ade4 makes base specific contacts with Asn167 and Lys226. Ade4 acts as the donor in it’s interaction with Lys226 and as the acceptor with Asn167. Ade6 interacts with Arg232 in which it acts as the donor.
Uracil recognition
Hrp1 interacts with the three uracil bases mainly though Van der Waals contacts. All three uridines interact with aromatic residues of the protein, although Ura3 and Ura5 have low surface accessibility compared to Ura7. Ura3 interacts with Phe288 in a nonplanar position, and Ura5 behaves the same with Phe162. However, Ura7 does form a planar stacking arrangement with the aromatic ring of Phe202. Base discrimination from cytosine relies heavily on the hydrogen bonding between the O4 of the uracil base and the amine group of Lys160. The O2 and O4 of Ura5 also play a role in base discrimination as they form hydrogen bonds with Lys244 and Lys231, respectively. While these two lysines are conserved in most Hrp1-like proteins, there are variations in other organisms that replace Lys244 with an Asn, although the interaction remains conserved. Discrimination at Ura3 is less clear, but it is suggested that it is due to the interaction of its N3 with the backbone phosphate of Ade6.
Novelty
While Trp168 is a highly conserved residue amongst fungal HRP1-like proteins, the tryptophan stacking found with Ade4 appears to be a novel feature not found in other single-stranded RNA and 2 RBD complexes. It is believed that Trp168 and the ring stacking it engages in are crucial to the affinity and base specificity for RNA.
To research Trp168's importance, studies have experimentally replaced it with phenylalanine and alanine side chains. While these mutants retained the ability to form 1 protein:1 RNA ratio complexes, the affinity for the AU repetition PEE is at least 10 times weaker than that of the wild-type. Furthermore, results of 15N-HSQC spectra comparisons suggest that while the Phe and Ala mutants share similar RNA-binding modes to each, they are completely different from the wild-type Trp.
 Figure 4:Trp168 "stacking" onto Ade4 through Van Der Waals interactions. Another structural finding of the splicing-factor Fox-1 in complex with RNA identifies Phe126 to have an equivalent position as Trp128 has in Hrp1. Studies were done to test the importance of Phe126 in RNA binding by mutating this residue. Similarly to the experiment mentioned above, it was concluded that the aromatic structure of Phe126 played an important role with affinity, as the residue engages in 2 planar stacking interactions with 2 RNA bases and makes contact with a third base. However, aromatic mutants did not have a significant effect on affinity, which suggests that they share a similar binding mode to the Phe126 wild-type. This is not the case with Trp168 in Hrp1, indicating that perhaps Hrp1 has strict sequence requirements.
Disease
Though Hrp1 is not analogous to any mammalian hnRNP[23], the protein and its corresponding gene are occasionally studied as orthologues to human hnRNPs. HNRPDL is one such family of human hnRNPs. Mutations to several members of this class of hnRNPs result in many facets of muscular dystrophy. A study by Vieira, et al. [24] found that elimination of Hrp1 had profound effects on protein localization and activation, and these results were used as a model for the genotypic causation of muscular dystrophy.
References
- ↑ Henry, Michael, et al. “Potential RNA Binding Proteins in Saccharomyces Cerevisiae Identified as Suppressors of Temperature-Sensitive Mutations inNPL3.” Genetics, vol. 142, Jan. 1996, pp. 103–115.
- ↑ Kessler, Marco M, et al. “Purification of the Saccharomyces Cerevisiae Cleavage/Polyadenylation Factor I.” Journal of Biological Chemistry, vol. 271, no. 43, 25 Oct. 1996, pp. 27167–27175.
- ↑ Guisbert, K. Kim. “Functional Specificity of Shuttling HnRNPs Revealed by Genome-Wide Analysis of Their RNA Binding Profiles.” RNA, vol. 11, no. 4, Jan. 2005, pp. 383–393., doi:10.1261/rna.7234205.
- ↑ Kessler, Marco M, et al. “Purification of the Saccharomyces Cerevisiae Cleavage/Polyadenylation Factor I.” Journal of Biological Chemistry, vol. 271, no. 43, 25 Oct. 1996, pp. 27167–27175.
- ↑ Minvielle-Sebastia, L. “Control of Cleavage Site Selection during MRNA 3 End Formation by a Yeast HnRNP.” The EMBO Journal, vol. 17, no. 24, 1998, pp. 7454–7468., doi:10.1093/emboj/17.24.7454.
- ↑ Kessler, M. M., et al. “Hrp1, a Sequence-Specific RNA-Binding Protein That Shuttles between the Nucleus and the Cytoplasm, Is Required for MRNA 3-End Formation in Yeast.” Genes & Development, vol. 11, no. 19, Jan. 1997, pp. 2545–2556., doi:10.1101/gad.11.19.2545.
- ↑ Barnwal, R. P., et al. “Structural and Biochemical Analysis of the Assembly and Function of the Yeast Pre-MRNA 3 End Processing Complex CF I.” Proceedings of the National Academy of Sciences, vol. 109, no. 52, Oct. 2012, pp. 21342–21347., doi:10.1073/pnas.1214102110.
- ↑ Barnwal, R. P., et al. “Structural and Biochemical Analysis of the Assembly and Function of the Yeast Pre-MRNA 3 End Processing Complex CF I.” Proceedings of the National Academy of Sciences, vol. 109, no. 52, Oct. 2012, pp. 21342–21347., doi:10.1073/pnas.1214102110.
- ↑ Leeper, Thomas C., et al. “Novel Protein–Protein Contacts Facilitate MRNA 3′-Processing Signal Recognition by Rna15 and Hrp1.” Journal of Molecular Biology, vol. 401, no. 3, 2010, pp. 334–349., doi:10.1016/j.jmb.2010.06.032.
- ↑ Guisbert, K. Kim. “Functional Specificity of Shuttling HnRNPs Revealed by Genome-Wide Analysis of Their RNA Binding Profiles.” RNA, vol. 11, no. 4, Jan. 2005, pp. 383–393., doi:10.1261/rna.7234205.
- ↑ Chen, S. “A Specific RNA-Protein Interaction at Yeast Polyadenylation Efficiency Elements.” Nucleic Acids Research, vol. 26, no. 21, Jan. 1998, pp. 4965–4974., doi:10.1093/nar/26.21.4965.
- ↑ Minvielle-Sebastia, L. “Control of Cleavage Site Selection during MRNA 3 End Formation by a Yeast HnRNP.” The EMBO Journal, vol. 17, no. 24, 1998, pp. 7454–7468., doi:10.1093/emboj/17.24.7454.
- ↑ Henry, M. F. “The Yeast HnRNP-like Protein Hrp1/Nab4 Accumulates in the Cytoplasm after Hyperosmotic Stress: A Novel Fps1-Dependent Response.” Molecular Biology of the Cell, vol. 14, no. 9, Nov. 2003, pp. 3929–3941., doi:10.1091/mbc.e03-01-0854.
- ↑ Henry, M. F. “The Yeast HnRNP-like Protein Hrp1/Nab4 Accumulates in the Cytoplasm after Hyperosmotic Stress: A Novel Fps1-Dependent Response.” Molecular Biology of the Cell, vol. 14, no. 9, Nov. 2003, pp. 3929–3941., doi:10.1091/mbc.e03-01-0854.
- ↑ Kessler, M. M., et al. “Hrp1, a Sequence-Specific RNA-Binding Protein That Shuttles between the Nucleus and the Cytoplasm, Is Required for MRNA 3-End Formation in Yeast.” Genes & Development, vol. 11, no. 19, Jan. 1997, pp. 2545–2556., doi:10.1101/gad.11.19.2545.
- ↑ Lange, Allison, et al. “A PY-NLS Nuclear Targeting Signal Is Required for Nuclear Localization and Function of TheSaccharomyces CerevisiaemRNA-Binding Protein Hrp1.” Journal of Biological Chemistry, vol. 283, no. 19, 2008, pp. 12926–12934., doi:10.1074/jbc.m800898200.
- ↑ Tuck, Alex C, and David Tollervey. “A Transcriptome-Wide Atlas of RNP Composition Reveals Diverse Classes of MRNAs and LncRNAs.” Cell, vol. 154, 29 Aug. 2013, pp. 996–1009.
- ↑ Kuehner, Jason N., and David A. Brow. “Regulation of a Eukaryotic Gene by GTP-Dependent Start Site Selection and Transcription Attenuation.” Molecular Cell, vol. 31, no. 2, 2008, pp. 201–211., doi:10.1016/j.molcel.2008.05.018.
- ↑ González, Carlos I., et al. “The Yeast HnRNP-like Protein Hrp1/Nab4 Marks a Transcript for Nonsense-Mediated MRNA Decay.” Molecular Cell, vol. 5, no. 3, 2000, pp. 489–499., doi:10.1016/s1097-2765(00)80443-8.
- ↑ Bucheli, M. E., et al. “Polyadenylation Site Choice in Yeast Is Affected by Competition between Npl3 and Polyadenylation Factor CFI.” RNA, vol. 13, no. 10, 2007, pp. 1756–1764., doi:10.1261/rna.607207.
- ↑ Clery, Antoine, et al. “RNA Recognition Motifs: Boring? Not Quite.” Current Opinion in Structural Biology, Elsevier Current Trends, 30 May 2008, www.sciencedirect.com/science/article/pii/S0959440X08000584.
- ↑ Perez-Canadillas, Jose Manuel. “Grabbing the Message: Structural Basis of MRNA 3â²UTR Recognition by Hrp1.” The EMBO Journal, vol. 25, no. 13, 2006, pp. 3167–3178., doi:10.1038/sj.emboj.7601190.
- ↑ Gross, S., and C. Moore. “Five Subunits Are Required for Reconstitution of the Cleavage and Polyadenylation Activities of Saccharomyces Cerevisiae Cleavage Factor I.” Proceedings of the National Academy of Sciences, vol. 98, no. 11, Aug. 2001, pp. 6080–6085., doi:10.1073/pnas.101046598.
- ↑ Vieira, Natássia M., et al. “A Defect in the RNA-Processing Protein HNRPDL Causes Limb-Girdle Muscular Dystrophy 1G (LGMD1G).” Human Molecular Genetics, vol. 23, no. 15, 2014, pp. 4103–4110., doi:10.1093/hmg/ddu127.
|




