We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Clayton Moore/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 2: | Line 2: | ||
== Introduction == | == Introduction == | ||
| - | Hrp1 is a heterogeneous ribonuclear protein of [https://en.wikipedia.org/wiki/Saccharomyces_cerevisiae Saccharomyces cerevisiae], baker’s yeast. Hrp1 is an essential component of 3’ [https://en.wikipedia.org/wiki/Post-transcriptional_modification pre-mRNA processing] and contributes to the preparatory cleavage required for polyadenylation. The gene expressed as Hrp1, HRP1, was first isolated by Henry, et al.<ref> Henry, Michael, et al. “Potential RNA Binding Proteins in Saccharomyces Cerevisiae Identified as Suppressors of Temperature-Sensitive Mutations inNPL3.” Genetics, vol. 142, Jan. 1996, pp. 103–115. </ref> and was later attributed to the Hrp1 protein by Kessler, et al.<ref name="Kessler 1996"> Kessler, Marco M, et al. “Purification of the Saccharomyces Cerevisiae Cleavage/Polyadenylation Factor I.” Journal of Biological Chemistry, vol. 271, no. 43, 25 Oct. 1996, pp. 27167–27175. </ref> Hrp1 also participates in the regulation of the 3’ end. | + | Hrp1 is a heterogeneous ribonuclear protein of [https://en.wikipedia.org/wiki/Saccharomyces_cerevisiae Saccharomyces cerevisiae], baker’s yeast. Hrp1 is an essential component of 3’ [https://en.wikipedia.org/wiki/Post-transcriptional_modification pre-mRNA processing] and contributes to the preparatory cleavage required for polyadenylation. The gene expressed as Hrp1, HRP1, was first isolated by Henry, et al.<ref> Henry, Michael, et al. “Potential RNA Binding Proteins in Saccharomyces Cerevisiae Identified as Suppressors of Temperature-Sensitive Mutations inNPL3.” Genetics, vol. 142, Jan. 1996, pp. 103–115. </ref> and was later attributed to the Hrp1 protein by Kessler, et al.<ref name="Kessler 1996"> Kessler, Marco M, et al. “Purification of the Saccharomyces Cerevisiae Cleavage/Polyadenylation Factor I.” Journal of Biological Chemistry, vol. 271, no. 43, 25 Oct. 1996, pp. 27167–27175. </ref> Hrp1 also participates in the regulation of the 3’ end. The structure was solved via NMR by Pérez-Cañadillas.</ref name="Perez-Canadillas 2006" /> |
<StructureSection load='2cjk' size='400' side='right' caption='(PDB entry [[2cjk]])' scene=''> | <StructureSection load='2cjk' size='400' side='right' caption='(PDB entry [[2cjk]])' scene=''> | ||
| Line 23: | Line 23: | ||
== Structure == | == Structure == | ||
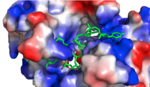
| - | HRP1 is made up of two [https://en.wikipedia.org/wiki/RNA RNA] binding domains (RBDs) that contain residues serving to facilitate RNA recognition. These two domains fold into a βαββαβ [https://en.wikipedia.org/wiki/Protein_secondary_structure secondary structure]<ref>Clery, Antoine, et al. “RNA Recognition Motifs: Boring? Not Quite.” Current Opinion in Structural Biology, Elsevier Current Trends, 30 May 2008, www.sciencedirect.com/science/article/pii/S0959440X08000584.</ref> in an RNA-free environment, allowing Hrp1 to behave rigidly. The <scene name='78/782604/First_rbd/4'>first RBD</scene> includes residues extending from Ser158 to Ala233 and the <scene name='78/782604/Second_rbd/2'>second RBD</scene> extends from Lys244 to Ala318. Both RBDs are composed of a four-stranded [https://en.wikipedia.org/wiki/Beta_sheet beta sheet] with two [https://en.wikipedia.org/wiki/Alpha_helix alpha helices] spanning across one side of the sheet. The linker region is made up of residues spanning from Ile234 to Gly243. When RNA is introduced into the environment, conformational change is demonstrated within the linker region and a <scene name='78/782604/Linker_helix/2'>short two-turn alpha helix</scene> forms from Arg236 to Lys241. The helix that forms is made up of many charged polar residues that [https://en.wikipedia.org/wiki/Salt_bridge_(protein_and_supramolecular) stablilize] themselves through <scene name='78/782604/Salt_bridges/2'>salt bridge interactions</scene> between Arg236-Asp240 and Asp237-Lys241.<ref | + | HRP1 is made up of two [https://en.wikipedia.org/wiki/RNA RNA] binding domains (RBDs) that contain residues serving to facilitate RNA recognition. These two domains fold into a βαββαβ [https://en.wikipedia.org/wiki/Protein_secondary_structure secondary structure]<ref>Clery, Antoine, et al. “RNA Recognition Motifs: Boring? Not Quite.” Current Opinion in Structural Biology, Elsevier Current Trends, 30 May 2008, www.sciencedirect.com/science/article/pii/S0959440X08000584.</ref> in an RNA-free environment, allowing Hrp1 to behave rigidly. The <scene name='78/782604/First_rbd/4'>first RBD</scene> includes residues extending from Ser158 to Ala233 and the <scene name='78/782604/Second_rbd/2'>second RBD</scene> extends from Lys244 to Ala318. Both RBDs are composed of a four-stranded [https://en.wikipedia.org/wiki/Beta_sheet beta sheet] with two [https://en.wikipedia.org/wiki/Alpha_helix alpha helices] spanning across one side of the sheet. The linker region is made up of residues spanning from Ile234 to Gly243. When RNA is introduced into the environment, conformational change is demonstrated within the linker region and a <scene name='78/782604/Linker_helix/2'>short two-turn alpha helix</scene> forms from Arg236 to Lys241. The helix that forms is made up of many charged polar residues that [https://en.wikipedia.org/wiki/Salt_bridge_(protein_and_supramolecular) stablilize] themselves through <scene name='78/782604/Salt_bridges/2'>salt bridge interactions</scene> between Arg236-Asp240 and Asp237-Lys241.<ref name="Perez-Canadillas 2006">Pérez-Cañadillas, Jose Manuel. “Grabbing the Message: Structural Basis of MRNA 3â²UTR Recognition by Hrp1.” The EMBO Journal, vol. 25, no. 13, 2006, pp. 3167–3178., doi:10.1038/sj.emboj.7601190. </ref> |
[[Image:Screen Shot 2018-04-02 at 9.57.35 PM.png |150px|left|thumb|'''Figure 1:'''Positively charged cleft within HRP1 in which RNA binds]] | [[Image:Screen Shot 2018-04-02 at 9.57.35 PM.png |150px|left|thumb|'''Figure 1:'''Positively charged cleft within HRP1 in which RNA binds]] | ||
Revision as of 17:53, 6 April 2018
HRP1 found in Saccharomyces cerevisiae
Introduction
Hrp1 is a heterogeneous ribonuclear protein of Saccharomyces cerevisiae, baker’s yeast. Hrp1 is an essential component of 3’ pre-mRNA processing and contributes to the preparatory cleavage required for polyadenylation. The gene expressed as Hrp1, HRP1, was first isolated by Henry, et al.[1] and was later attributed to the Hrp1 protein by Kessler, et al.[2] Hrp1 also participates in the regulation of the 3’ end. The structure was solved via NMR by Pérez-Cañadillas.</ref name="Perez-Canadillas 2006" />
| |||||||||||