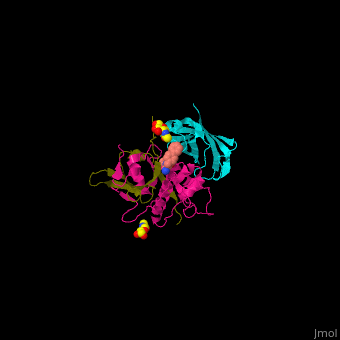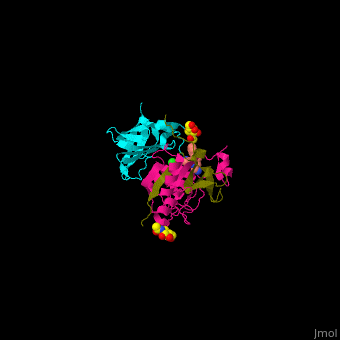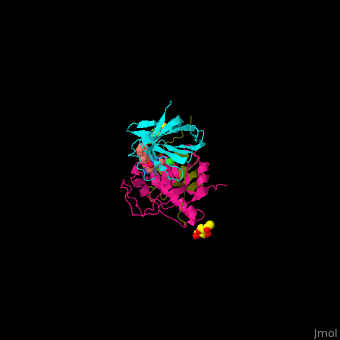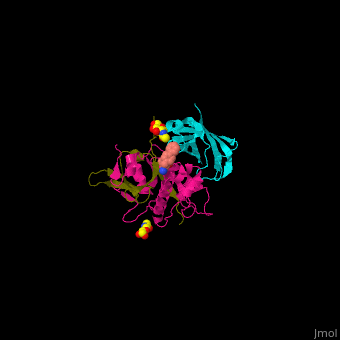Dipeptidyl peptidase
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | <StructureSection load=' | + | <StructureSection load='' size='350' side='right' caption='Human glycosylated dipeptidyl peptidase I complex with inhibitor, glycerol and chloride ion (green) (PDB entry [[4cdc]])' scene='43/433126/Cv/3'> |
__TOC__ | __TOC__ | ||
== Function == | == Function == | ||
Revision as of 13:04, 1 May 2018
| |||||||||||
3D Structures of Dipeptidyl peptidase
Updated on 01-May-2018
References
- ↑ Sakamoto Y, Suzuki Y, Iizuka I, Tateoka C, Roppongi S, Fujimoto M, Inaka K, Tanaka H, Yamada M, Ohta K, Gouda H, Nonaka T, Ogasawara W, Tanaka N. Structural and mutational analyses of dipeptidyl peptidase 11 from Porphyromonas gingivalis reveal the molecular basis for strict substrate specificity. Sci Rep. 2015 Jun 9;5:11151. doi: 10.1038/srep11151. PMID:26057589 doi:http://dx.doi.org/10.1038/srep11151
- ↑ Pro B, Dang NH. CD26/dipeptidyl peptidase IV and its role in cancer. Histol Histopathol. 2004 Oct;19(4):1345-51. PMID:15375776
- ↑ Barnett A. DPP-4 inhibitors and their potential role in the management of type 2 diabetes. Int J Clin Pract. 2006 Nov;60(11):1454-70. PMID:17073841 doi:10.1111/j.1742-1241.2006.01178.x
- ↑ Itou M, Kawaguchi T, Taniguchi E, Sata M. Dipeptidyl peptidase-4: a key player in chronic liver disease. World J Gastroenterol. 2013 Apr 21;19(15):2298-306. doi:, 10.3748/wjg.v19.i15.2298. PMID:23613622 doi:http://dx.doi.org/10.3748/wjg.v19.i15.2298
- ↑ Furber M, Tiden AK, Gardiner P, Mete A, Ford R, Millichip I, Stein L, Mather A, Kinchin E, Luckhurst C, Barber S, Cage P, Sanganee H, Austin R, Chohan K, Beri R, Thong B, Wallace A, Oreffo V, Hutchinson R, Harper S, Debreczeni J, Breed J, Wissler L, Edman K. Cathepsin C Inhibitors: Property Optimization and Identification of a Clinical Candidate. J Med Chem. 2014 Mar 14. PMID:24592859 doi:http://dx.doi.org/10.1021/jm401705g