Sandbox Reserved 1506
From Proteopedia
| Line 1: | Line 1: | ||
<Structure load='Insert PDB code or filename here' size='350' frame='true' align='right' caption='Insert caption here' scene='Insert optional scene name here' /><scene name='80/802680/Anaat/1'>ANAAT en couleur</scene> | <Structure load='Insert PDB code or filename here' size='350' frame='true' align='right' caption='Insert caption here' scene='Insert optional scene name here' /><scene name='80/802680/Anaat/1'>ANAAT en couleur</scene> | ||
'<Structure load='1b6b' size='350' frame='true' align='right' caption='Protein Display ' scene='Insert optional scene name here' />'{{Sandbox_Reserved_ESBS}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | '<Structure load='1b6b' size='350' frame='true' align='right' caption='Protein Display ' scene='Insert optional scene name here' />'{{Sandbox_Reserved_ESBS}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | ||
| - | + | ||
This is a default text for your page '''b6'''. Click above on '''edit this page''' to modify. Be careful with the < and > signs. | This is a default text for your page '''b6'''. Click above on '''edit this page''' to modify. Be careful with the < and > signs. | ||
| Line 9: | Line 9: | ||
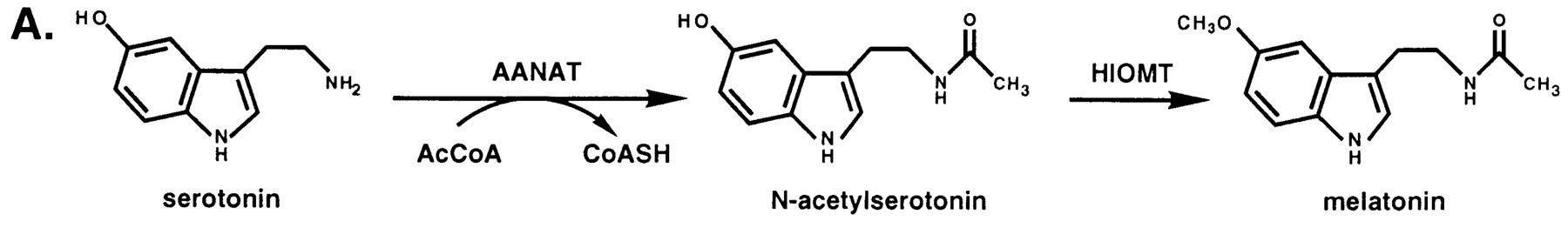
Serotonin N-acetyltransferase is also named aralkylamine-N-acetyltransferase (ANAAT). This enzyme catalyzes the acetylation of the amine group on serotonin, an intermediate in melatonin synthesis. It forms the penultimate enzyme in the melatonin pathway, as it is shown on fig1. | Serotonin N-acetyltransferase is also named aralkylamine-N-acetyltransferase (ANAAT). This enzyme catalyzes the acetylation of the amine group on serotonin, an intermediate in melatonin synthesis. It forms the penultimate enzyme in the melatonin pathway, as it is shown on fig1. | ||
| - | + | Circulating melatonin plays a role in the circadian rythm. Day/night differences in circulating melatonin levels provide a hormonal analog signal of environmental lighting, which is used in a variety of ways to optimize circadian and circannual rhythms in physiology[2]. The | |
[[Image:melationin synthesis.jpg]] | [[Image:melationin synthesis.jpg]] | ||
| + | |||
| + | == Structure == | ||
| + | |||
== Regulation == | == Regulation == | ||
| Line 23: | Line 26: | ||
[1] Hickman, A. B; Klein, D. C; Dyda, F. Melatonin Biosynthesis: The Structure of Serotonin N-Acetyltransferase at 2.5A Resolution Suggests a Catalytic Mechanism. Mol. Cell. 1999, 3-1, 23-32. | [1] Hickman, A. B; Klein, D. C; Dyda, F. Melatonin Biosynthesis: The Structure of Serotonin N-Acetyltransferase at 2.5A Resolution Suggests a Catalytic Mechanism. Mol. Cell. 1999, 3-1, 23-32. | ||
| - | [2] Melatonin synthesis: 14-3-3-dependent activation and inhibition of arylalkylamine N -acetyltransferase mediated by phosphoserine-205 - Surajit Ganguly, Joan L. Weller, Anthony Ho, Philippe Chemineau, Benoit Malpaux, and David C. Klein - PNAS, January 25, 2005. | + | [2] Arendt, J. (1995) Melatonin and the Mammalian Pineal Gland (Chapman & Hall, London), pp. 201–285. |
| + | |||
| + | [3] Melatonin synthesis: 14-3-3-dependent activation and inhibition of arylalkylamine N -acetyltransferase mediated by phosphoserine-205 - Surajit Ganguly, Joan L. Weller, Anthony Ho, Philippe Chemineau, Benoit Malpaux, and David C. Klein - PNAS, January 25, 2005. | ||
| - | [ | + | [4] The Structural Basis of Ordered Substrate Binding by Serotonin N-Acetyltransferase: Enzyme Complex at 1.8 A ˚ Resolution with a Bisubstrate Analog - Alison Burgess Hickman,M. A. A. Namboodiri, David C. Klein, and Fred Dyda - Cell, Vol. 97, 361–369, April 30, 1999. |
Revision as of 12:44, 2 January 2019
|
|
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |
This is a default text for your page b6. Click above on edit this page to modify. Be careful with the < and > signs.
You may include any references to papers as in: the use of JSmol in Proteopedia [1] or to the article describing Jmol [2] to the rescue.
Contents |
Function
Serotonin N-acetyltransferase is also named aralkylamine-N-acetyltransferase (ANAAT). This enzyme catalyzes the acetylation of the amine group on serotonin, an intermediate in melatonin synthesis. It forms the penultimate enzyme in the melatonin pathway, as it is shown on fig1. Circulating melatonin plays a role in the circadian rythm. Day/night differences in circulating melatonin levels provide a hormonal analog signal of environmental lighting, which is used in a variety of ways to optimize circadian and circannual rhythms in physiology[2]. The
Structure
Regulation
Relevance
Structural highlights
</StructureSection>
References
[1] Hickman, A. B; Klein, D. C; Dyda, F. Melatonin Biosynthesis: The Structure of Serotonin N-Acetyltransferase at 2.5A Resolution Suggests a Catalytic Mechanism. Mol. Cell. 1999, 3-1, 23-32.
[2] Arendt, J. (1995) Melatonin and the Mammalian Pineal Gland (Chapman & Hall, London), pp. 201–285.
[3] Melatonin synthesis: 14-3-3-dependent activation and inhibition of arylalkylamine N -acetyltransferase mediated by phosphoserine-205 - Surajit Ganguly, Joan L. Weller, Anthony Ho, Philippe Chemineau, Benoit Malpaux, and David C. Klein - PNAS, January 25, 2005.
[4] The Structural Basis of Ordered Substrate Binding by Serotonin N-Acetyltransferase: Enzyme Complex at 1.8 A ˚ Resolution with a Bisubstrate Analog - Alison Burgess Hickman,M. A. A. Namboodiri, David C. Klein, and Fred Dyda - Cell, Vol. 97, 361–369, April 30, 1999.
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644