Sandbox Reserved 1508
From Proteopedia
| Line 1: | Line 1: | ||
{{Sandbox_Reserved_ESBS}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | {{Sandbox_Reserved_ESBS}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | ||
| - | ==The protein 5C04== | + | =='''The protein 5C04''' |
| + | == Headline text == | ||
| + | == | ||
<StructureSection load='5C04' size='340' side='right' caption='Caption for this structure' scene=''> | <StructureSection load='5C04' size='340' side='right' caption='Caption for this structure' scene=''> | ||
<div align="justified">The protein 5C04 is classified as an oxidoreductase. We found it in the ''Mycobacterium tuberculosis'' organism, especially in the strain ATCC 25618/H37Rv. It can be expressed in ''Escherichia Coli'' bacteria. This is a pathogenic protein which is involved in the tuberculosis. Its pathogenicity is due to a specific mutation in the active site of peroxiredoxins.</div> | <div align="justified">The protein 5C04 is classified as an oxidoreductase. We found it in the ''Mycobacterium tuberculosis'' organism, especially in the strain ATCC 25618/H37Rv. It can be expressed in ''Escherichia Coli'' bacteria. This is a pathogenic protein which is involved in the tuberculosis. Its pathogenicity is due to a specific mutation in the active site of peroxiredoxins.</div> | ||
| Line 32: | Line 34: | ||
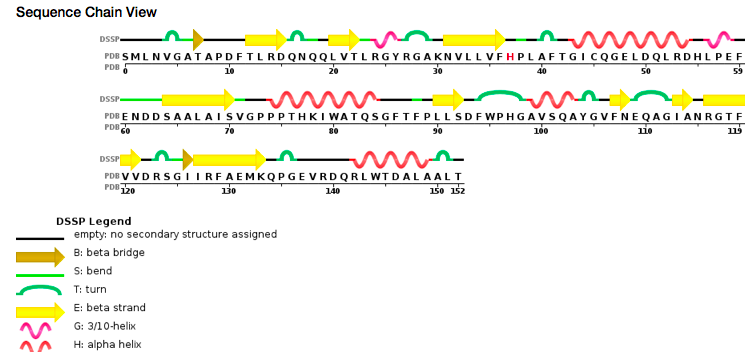
| - | At the active site of the enzyme, a pyrodoxal-phosphate cofactor is covalently linked to the Lysine 51, an invariant residue. A parallel β-sheet associated with three α-helices are part of the N terminal domain (residues 46 to 153). Two of those α-helices are part of the dimer interface and the third one is partly forming the entrance of the active site as on the other side of the β-sheet. On the other hand the C-terminal domain is made of 6-stranded mixed β-sheets surrounded by four α-helices (two on each sides) and of residues with a unique insertion of eight amino acids within them. (Ågren et al, 2008) | + | At the active site of the enzyme, a pyrodoxal-phosphate cofactor is covalently linked to the Lysine 51 <scene name='80/802682/Lys_51/1'>Lys 51</scene>, an invariant residue. A parallel β-sheet associated with three α-helices are part of the N terminal domain (residues 46 to 153). Two of those α-helices are part of the dimer interface and the third one is partly forming the entrance of the active site as on the other side of the β-sheet. On the other hand the C-terminal domain is made of 6-stranded mixed β-sheets surrounded by four α-helices (two on each sides) and of residues with a unique insertion of eight amino acids within them. (Ågren et al, 2008) |
All those compounds allow the enzyme to have several conformations : an open one, a closed one (when a substrate is bound to the enzyme) and an inhibited form (when there is a chlorite bound at an allosteric site). (Ågren et al, 2008) | All those compounds allow the enzyme to have several conformations : an open one, a closed one (when a substrate is bound to the enzyme) and an inhibited form (when there is a chlorite bound at an allosteric site). (Ågren et al, 2008) | ||
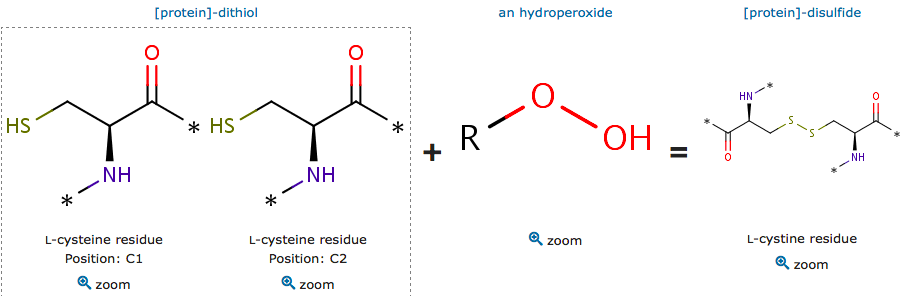
| - | The cysteine are polar uncharged amino acids. It has the particularity to be easily oxidized to form a dimer containing disulfide bridge between two cysteine. Important protein nonpolar residues in the dimer interface have been shown. The proximity between this hydrophobic region and Cys residues allows this kind of substrates to lay most of their aliphatic carbon chains over the patch, supporting the direct interaction of the peroxide group with the reactive thiolate group (Zeida et al, 2015). There is a complex hydrogen bound network which is involved in the Thr and oxygen bonding. | + | The cysteine are polar uncharged amino acids. It has the particularity to be easily oxidized to form a dimer containing disulfide bridge between two cysteine. Important protein nonpolar residues in the dimer interface have been shown. The proximity between this hydrophobic region and Cys residues allows this kind of substrates to lay most of their aliphatic carbon chains over the patch, supporting the direct interaction of the peroxide group with the reactive thiolate group (Zeida et al, 2015). There is a complex hydrogen bound network which is involved in the Thr and oxygen bonding. |
Additionally, there is fatty acid, derived from hydroperoxide, involved in the reduction of the H2O2. Peroxidase involves a proton transfer from the both oxygens that occurs after transtion state. | Additionally, there is fatty acid, derived from hydroperoxide, involved in the reduction of the H2O2. Peroxidase involves a proton transfer from the both oxygens that occurs after transtion state. | ||
The oxidized reactive cystein have an unprotonated form of sulfenic acid and a protonated form. The reduction mechanism of these subtrate is the same as for H2O2. | The oxidized reactive cystein have an unprotonated form of sulfenic acid and a protonated form. The reduction mechanism of these subtrate is the same as for H2O2. | ||
Revision as of 14:43, 11 January 2019
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |
==The protein 5C04
Headline text
==
| |||||||||||
References
Ågren, Daniel, Robert Schnell, Wulf Oehlmann, Mahavir Singh, et Gunter Schneider. « Cysteine Synthase (CysM) of Mycobacterium Tuberculosis Is an O -Phosphoserine Sulfhydrylase: EVIDENCE FOR AN ALTERNATIVE CYSTEINE BIOSYNTHESIS PATHWAY IN MYCOBACTERIA ». Journal of Biological Chemistry 283, nᵒ 46 (14 novembre 2008): 31567‑74. https://doi.org/10.1074/jbc.M804877200.
Burns, Kristin E., Sabine Baumgart, Pieter C. Dorrestein, Huili Zhai, Fred W. McLafferty, et Tadhg P. Begley. « Reconstitution of a New Cysteine Biosynthetic Pathway in Mycobacterium t Uberculosis ». Journal of the American Chemical Society 127, nᵒ 33 (août 2005): 11602‑3. https://doi.org/10.1021/ja053476x.
Pedre, Brandán, Laura A. H. van Bergen, Anna Palló, Leonardo A. Rosado, Veronica Tamu Dufe, Inge Van Molle, Khadija Wahni, et al. « The Active Site Architecture in Peroxiredoxins: A Case Study on Mycobacterium Tuberculosis AhpE ». Chemical Communications 52, nᵒ 67 (2016): 10293‑96. https://doi.org/10.1039/C6CC02645A.
Rhee, Sue Goo, et Hyun Ae Woo. « Multiple Functions of Peroxiredoxins: Peroxidases, Sensors and Regulators of the Intracellular Messenger H 2 O 2 , and Protein Chaperones ». Antioxidants & Redox Signaling 15, nᵒ 3 (août 2011): 781‑94. https://doi.org/10.1089/ars.2010.3393.
Zeida, Ari, Aníbal M. Reyes, Pablo Lichtig, Martín Hugo, Diego S. Vazquez, Javier Santos, F. Luis González Flecha, Rafael Radi, Dario A. Estrin, et Madia Trujillo. « Molecular Basis of Hydroperoxide Specificity in Peroxiredoxins: The Case of AhpE from Mycobacterium Tuberculosis ». Biochemistry 54, nᵒ 49 (15 décembre 2015): 7237‑47. https://doi.org/10.1021/acs.biochem.5b00758.