User:Ashley Crotteau/Sandbox1
From Proteopedia
(Difference between revisions)
| Line 16: | Line 16: | ||
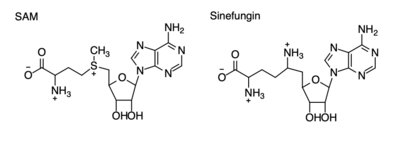
The SET domain is mostly defined by <scene name='81/811707/Variable_knot/2'>turns and loops</scene> with the few <scene name='81/811707/Beta_sheets/3'>antiparallel β-sheets</scene>.<ref name="Schubert" /> <scene name='81/811707/Beta-hairpin/3'>Residues 337-349</scene> form a β-hairpin that sticks out at a right angle to the surface of the enzyme.<ref name="Xiao" /> The following three residues (<scene name='81/811707/Sharp_bend/3'>350-352</scene>) accommodate a sharp bend in the peptide chain and the end of the protein adopts an <scene name='81/811707/C-term_alpha_helix/2'>α-helical conformation</scene>.<ref name="Xiao" /> The two most defining features of the SET domain are the C-terminal tyrosine and the knot-like fold. These two components have been recognized to be essential for S-adenosyl-L-methionine (SAM) binding and catalysis.<ref name="Schubert" /> <ref name="Yeates" /> <ref name="Huang" /> The knot-like fold contains the binding sites for the cofactor SAM and the peptide substrate.<ref name="Licciardello" /> | The SET domain is mostly defined by <scene name='81/811707/Variable_knot/2'>turns and loops</scene> with the few <scene name='81/811707/Beta_sheets/3'>antiparallel β-sheets</scene>.<ref name="Schubert" /> <scene name='81/811707/Beta-hairpin/3'>Residues 337-349</scene> form a β-hairpin that sticks out at a right angle to the surface of the enzyme.<ref name="Xiao" /> The following three residues (<scene name='81/811707/Sharp_bend/3'>350-352</scene>) accommodate a sharp bend in the peptide chain and the end of the protein adopts an <scene name='81/811707/C-term_alpha_helix/2'>α-helical conformation</scene>.<ref name="Xiao" /> The two most defining features of the SET domain are the C-terminal tyrosine and the knot-like fold. These two components have been recognized to be essential for S-adenosyl-L-methionine (SAM) binding and catalysis.<ref name="Schubert" /> <ref name="Yeates" /> <ref name="Huang" /> The knot-like fold contains the binding sites for the cofactor SAM and the peptide substrate.<ref name="Licciardello" /> | ||
| - | ===Active Site and Channel=== | ||
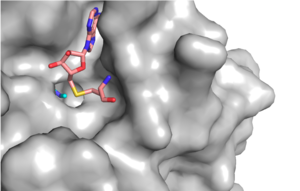
[[Image:KMT_Channel.png|300 px|right|thumb|Figure 1: Narrow Lysine Channel]] | [[Image:KMT_Channel.png|300 px|right|thumb|Figure 1: Narrow Lysine Channel]] | ||
| + | ===Active Site and Channel=== | ||
| + | |||
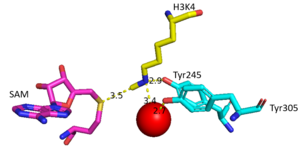
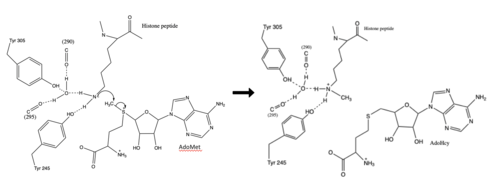
The most notable feature of the HKMT is the presence of the lysine access channel as the active site. The cofactor and <scene name='81/811708/Sam_structure/3'>peptide</scene> substrate are actually located on opposite sides of the SET domain but are connected through this narrow channel (Figure 1).<ref name="Xiao" /> This channel allows these two components to interact and complete the methyltransfer. The active site in general is considerably tyrosine rich. Residues Tyr245, His297, Ser268, Tyr305, Tyr335, and Tyr337 all help to shape the active site and the channel.<ref name="Xiao" /> The cofactor involved, SAM, provides the methyl for methylation of the lysine on its sulfur atom. | The most notable feature of the HKMT is the presence of the lysine access channel as the active site. The cofactor and <scene name='81/811708/Sam_structure/3'>peptide</scene> substrate are actually located on opposite sides of the SET domain but are connected through this narrow channel (Figure 1).<ref name="Xiao" /> This channel allows these two components to interact and complete the methyltransfer. The active site in general is considerably tyrosine rich. Residues Tyr245, His297, Ser268, Tyr305, Tyr335, and Tyr337 all help to shape the active site and the channel.<ref name="Xiao" /> The cofactor involved, SAM, provides the methyl for methylation of the lysine on its sulfur atom. | ||
Revision as of 18:39, 5 April 2019
H. sapiens Lysine Methyltransferase, SET 7/9
| |||||||||||
References
[3] [5] [1] [2] [4] [8] [6] [7]
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Schubert HL, Blumenthal RM, Cheng X. Many paths to methyltransfer: a chronicle of convergence. Trends Biochem Sci. 2003 Jun;28(6):329-35. PMID:12826405
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 Yeates TO. Structures of SET domain proteins: protein lysine methyltransferases make their mark. Cell. 2002 Oct 4;111(1):5-7. PMID:12372294
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 Xiao B, Jing C, Wilson JR, Walker PA, Vasisht N, Kelly G, Howell S, Taylor IA, Blackburn GM, Gamblin SJ. Structure and catalytic mechanism of the human histone methyltransferase SET7/9. Nature. 2003 Feb 6;421(6923):652-6. Epub 2003 Jan 22. PMID:12540855 doi:10.1038/nature01378
- ↑ 4.0 4.1 Huang S, Shao G, Liu L. The PR domain of the Rb-binding zinc finger protein RIZ1 is a protein binding interface and is related to the SET domain functioning in chromatin-mediated gene expression. J Biol Chem. 1998 Jun 26;273(26):15933-9. PMID:9632640
- ↑ 5.0 5.1 doi: https://dx.doi.org/10.1016/C2014-0-02189-2
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 Del Rizzo PA, Couture JF, Dirk LM, Strunk BS, Roiko MS, Brunzelle JS, Houtz RL, Trievel RC. SET7/9 catalytic mutants reveal the role of active site water molecules in lysine multiple methylation. J Biol Chem. 2010 Oct 8;285(41):31849-58. Epub 2010 Aug 1. PMID:20675860 doi:http://dx.doi.org/10.1074/jbc.M110.114587
- ↑ 7.0 7.1 7.2 7.3 7.4 Sun G, Reddy MA, Yuan H, Lanting L, Kato M, Natarajan R. Epigenetic histone methylation modulates fibrotic gene expression. J Am Soc Nephrol. 2010 Dec;21(12):2069-80. doi: 10.1681/ASN.2010060633. Epub 2010, Oct 7. PMID:20930066 doi:http://dx.doi.org/10.1681/ASN.2010060633
- ↑ https://en.wikipedia.org/wiki/SET_domain#Structure
Student Contributors
Ashley Crotteau
Parker Hiday
Lauren Allman