User:Ashley Crotteau/Sandbox1
From Proteopedia
(Difference between revisions)
| Line 5: | Line 5: | ||
==Introduction== | ==Introduction== | ||
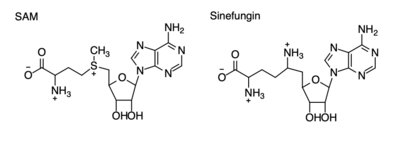
| - | [https://en.wikipedia.org/wiki/Histone Histones] are a family of proteins that condense DNA into chromatin, and is an octamer composed of two of each protein core; H2A, H2B, H3, and H4. Histones are a globular protein, that often have N- or C-terminal tails. These tails can often be subjected to modifications by enzymes. Methylation of histones is one of the four common histone modifications. Methylation is most common on long tails of H3 and H4 due to the tail being able to enter the active site.<ref name ="DesJarlais" /> A histone can be mono-, di-, or tri- methylated. Once the histone is methylated, the DNA goes from tightly bound heterochromatin to loosely packed euchromatin. The euchromatin allows RNA pol II to bind to the DNA and start transcription. <ref name="Marino" /> Histone methylation is also a major epigenetic marker, which can be passed down from generation to generation. A epigenetic marker affects the way that genes are expressed, and can either activate or repress DNA. Histone methylation is a major epigenetic marker because it has the ability to change heterochromatin to euchromatin, and vise versa. Alterations in markers have been associated with many diseases.<ref name="Xiao" /> Lysine Methyltrasferase SET7/9 (KMT) is an enzyme that methylates the histone 3 lysine 4 (H3K4) and plays a important role in the transcription of DNA in H. sapiens. | + | [https://en.wikipedia.org/wiki/Histone Histones] are a family of proteins that condense DNA into chromatin, and is an octamer composed of two of each protein core; H2A, H2B, H3, and H4. Histones are a globular protein, that often have N- or C-terminal tails. These tails can often be subjected to modifications by enzymes. Methylation of histones is one of the four common histone modifications. Methylation is most common on long tails of H3 and H4 due to the tail being able to enter the active site.<ref name ="DesJarlais" /> A histone can be mono-, di-, or tri- methylated. Once the histone is methylated, the DNA goes from tightly bound heterochromatin to loosely packed euchromatin. The euchromatin allows RNA pol II to bind to the DNA and start transcription. <ref name="Marino" /> Histone methylation is also a major epigenetic marker, which can be passed down from generation to generation. A epigenetic marker affects the way that genes are expressed, and can either activate or repress DNA. Histone methylation is a major epigenetic marker because it has the ability to change heterochromatin to euchromatin, and vise versa. Alterations in markers have been associated with many diseases.<ref name="Xiao" /> Lysine Methyltrasferase SET7/9 (KMT) is an enzyme that methylates the histone 3 lysine 4 (H3K4) and plays a important role in the transcription of DNA in H. sapiens.<ref name="Biterge" /> It is composed of 259 residues and is a monomer containing a SET domain. There is a two-domain architecture containing a conserved anti-parallel β-barrel and an unusual knot-like structure that creates the active site. It also contains a cofactor that plays a role in the active site.The methylation of H3K4 results in transcriptional activation.<ref name="Biterge" /> The specific methylation of H3K4 does not result in a change in charge because it is a nonpolar group being added to the lysine. A change in charge could result in tighter bound heterochromatin. |
| Line 28: | Line 28: | ||
== Function == | == Function == | ||
| - | |||
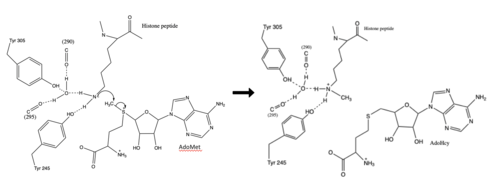
[[Image:KMT Mechanism .png|500 px|right|thumb|Figure 3: Histone Methylation by HKMT Mechanism]] | [[Image:KMT Mechanism .png|500 px|right|thumb|Figure 3: Histone Methylation by HKMT Mechanism]] | ||
| + | |||
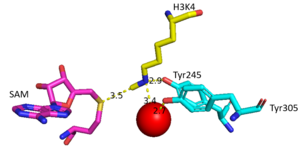
| + | The N from the lysine serves as a nucleophile that attacks the electrophilic CH<sub>3</sub> that is present in the AdoMet. The sulfur that the CH<sub>3</sub> is attached to pulls the electrons towards itself to weaken the bond between the sulfur and the carbon. This weak bond allows for the N to break that bond and take the methyl group. The N on the lysine is being stabilized by Tyr residues and a water (Figure 3). This allows the N to gain the methyl and take up that positive charge. | ||
The conserved regions of KMT has been found to be vital for function. Multiple studies have found that a mutation to any of the conserved tyrosine residues continues to monomethylate SAM, while it also creates di-methylation and tri- methylation of SAM.<ref name="Del Rizzo" /> In a recent study, Y245A and Y305F were created through site-directed mutagenesis.<ref name="Del Rizzo" /> Due to size, Ala245 was found to create a larger opening of the channel than tyrosine, which allowed for further methylation of SAM. <ref name="Del Rizzo" /> However, Y305F also showed the same characteristics of di- and tri-methylation, most likely due to a decrease of tyrosine residue interaction with water.<ref name="Del Rizzo" /> As there are four invariant conserved tyrosine residues (Tyr305, Tyr245, Tyr335, Tyr337) in the active site, this finding indicates that the function of KMT is dependent on the presence of tyrosine residues in the active site. <ref name="Del Rizzo" /> | The conserved regions of KMT has been found to be vital for function. Multiple studies have found that a mutation to any of the conserved tyrosine residues continues to monomethylate SAM, while it also creates di-methylation and tri- methylation of SAM.<ref name="Del Rizzo" /> In a recent study, Y245A and Y305F were created through site-directed mutagenesis.<ref name="Del Rizzo" /> Due to size, Ala245 was found to create a larger opening of the channel than tyrosine, which allowed for further methylation of SAM. <ref name="Del Rizzo" /> However, Y305F also showed the same characteristics of di- and tri-methylation, most likely due to a decrease of tyrosine residue interaction with water.<ref name="Del Rizzo" /> As there are four invariant conserved tyrosine residues (Tyr305, Tyr245, Tyr335, Tyr337) in the active site, this finding indicates that the function of KMT is dependent on the presence of tyrosine residues in the active site. <ref name="Del Rizzo" /> | ||
Revision as of 15:45, 12 April 2019
H. sapiens Lysine Methyltransferase, SET 7/9
| |||||||||||
References
[3] [8] [5] [6] [7] [9] [10] [11] [1] [2] [4]
- ↑ 1.0 1.1 DesJarlais R, Tummino PJ. Role of Histone-Modifying Enzymes and Their Complexes in Regulation of Chromatin Biology. Biochemistry. 2016 Mar 22;55(11):1584-99. doi: 10.1021/acs.biochem.5b01210. Epub , 2016 Jan 26. PMID:26745824 doi:http://dx.doi.org/10.1021/acs.biochem.5b01210
- ↑ 2.0 2.1 Marino-Ramirez L, Kann MG, Shoemaker BA, Landsman D. Histone structure and nucleosome stability. Expert Rev Proteomics. 2005 Oct;2(5):719-29. PMID:16209651 doi:http://dx.doi.org/10.1586/14789450.2.5.719
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 3.9 Xiao B, Jing C, Wilson JR, Walker PA, Vasisht N, Kelly G, Howell S, Taylor IA, Blackburn GM, Gamblin SJ. Structure and catalytic mechanism of the human histone methyltransferase SET7/9. Nature. 2003 Feb 6;421(6923):652-6. Epub 2003 Jan 22. PMID:12540855 doi:10.1038/nature01378
- ↑ 4.0 4.1 4.2 doi: https://dx.doi.org/10.15406/mojcsr.2016.03.00047
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 Schubert HL, Blumenthal RM, Cheng X. Many paths to methyltransfer: a chronicle of convergence. Trends Biochem Sci. 2003 Jun;28(6):329-35. PMID:12826405
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 Yeates TO. Structures of SET domain proteins: protein lysine methyltransferases make their mark. Cell. 2002 Oct 4;111(1):5-7. PMID:12372294
- ↑ 7.0 7.1 Huang S, Shao G, Liu L. The PR domain of the Rb-binding zinc finger protein RIZ1 is a protein binding interface and is related to the SET domain functioning in chromatin-mediated gene expression. J Biol Chem. 1998 Jun 26;273(26):15933-9. PMID:9632640
- ↑ 8.0 8.1 doi: https://dx.doi.org/10.1016/C2014-0-02189-2
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 Del Rizzo PA, Couture JF, Dirk LM, Strunk BS, Roiko MS, Brunzelle JS, Houtz RL, Trievel RC. SET7/9 catalytic mutants reveal the role of active site water molecules in lysine multiple methylation. J Biol Chem. 2010 Oct 8;285(41):31849-58. Epub 2010 Aug 1. PMID:20675860 doi:http://dx.doi.org/10.1074/jbc.M110.114587
- ↑ 10.0 10.1 10.2 10.3 10.4 Sun G, Reddy MA, Yuan H, Lanting L, Kato M, Natarajan R. Epigenetic histone methylation modulates fibrotic gene expression. J Am Soc Nephrol. 2010 Dec;21(12):2069-80. doi: 10.1681/ASN.2010060633. Epub 2010, Oct 7. PMID:20930066 doi:http://dx.doi.org/10.1681/ASN.2010060633
- ↑ 11.0 11.1 Tian X, Zhang S, Liu HM, Zhang YB, Blair CA, Mercola D, Sassone-Corsi P, Zi X. Histone lysine-specific methyltransferases and demethylases in carcinogenesis: new targets for cancer therapy and prevention. Curr Cancer Drug Targets. 2013 Jun;13(5):558-79. doi:, 10.2174/1568009611313050007. PMID:23713993 doi:http://dx.doi.org/10.2174/1568009611313050007
Student Contributors
Ashley Crotteau
Parker Hiday
Lauren Allman