We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1482
From Proteopedia
(Difference between revisions)
| Line 56: | Line 56: | ||
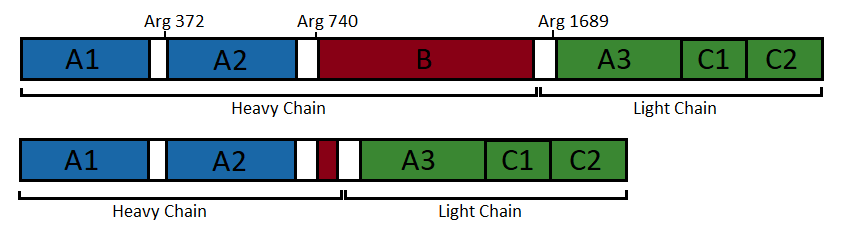
• The <scene name='80/802656/Light_chain/2'>light chain</scene> has a molecular weight of 80 kDa and is composed of 684 amino acids <ref name="Binhoreau" />. It contains two domains: a unique A domain of 371 amino acids and a duplicated C domain of 153 amino acids and 160 amino acids, respectively <ref name="Binhoreau" />. These domains are ranked in the following order A<sub>3</sub>-C<sub>1</sub>-C<sub>2</sub> <ref name="wikipedia" /><ref name="Binhoreau" />. It is composed of 42 % irregular structure, 36 % β-strands, and 22 % α-helices <ref name="Binhoreau" />. The C<sub>1</sub> and C<sub>2</sub> domains are defined by a distorted β barrel, while A<sub>3</sub>, as well as A<sub>1</sub> and A<sub>2</sub>, is composed of two connected β barrels <ref name="Ngo" />. This chain also contains of the major binding site of von Willebrand Factor at its N-terminus <ref name="Binhoreau" />. | • The <scene name='80/802656/Light_chain/2'>light chain</scene> has a molecular weight of 80 kDa and is composed of 684 amino acids <ref name="Binhoreau" />. It contains two domains: a unique A domain of 371 amino acids and a duplicated C domain of 153 amino acids and 160 amino acids, respectively <ref name="Binhoreau" />. These domains are ranked in the following order A<sub>3</sub>-C<sub>1</sub>-C<sub>2</sub> <ref name="wikipedia" /><ref name="Binhoreau" />. It is composed of 42 % irregular structure, 36 % β-strands, and 22 % α-helices <ref name="Binhoreau" />. The C<sub>1</sub> and C<sub>2</sub> domains are defined by a distorted β barrel, while A<sub>3</sub>, as well as A<sub>1</sub> and A<sub>2</sub>, is composed of two connected β barrels <ref name="Ngo" />. This chain also contains of the major binding site of von Willebrand Factor at its N-terminus <ref name="Binhoreau" />. | ||
| - | Figure 1: Domain organisation of the uncleaved coagulation factor VIII (top) and the engineered factor without B domain (bottom) | ||
| + | Figure 1: Domain organisation of the uncleaved coagulation factor VIII (top) and the engineered factor without B domain (bottom). (Figure adapted from <ref name="Ngo" />) | ||
| + | [[Image:Domain organisation factorVIII (un)cleaved.png]] | ||
Both chains are non-covalently associated through to a calcium ion to form the active heterodimer <ref name="Ngo" /><ref name="Binhoreau" />. This complex is the pro-coagulant factor VIIIa <ref name="wikipedia" />. | Both chains are non-covalently associated through to a calcium ion to form the active heterodimer <ref name="Ngo" /><ref name="Binhoreau" />. This complex is the pro-coagulant factor VIIIa <ref name="wikipedia" />. | ||
Revision as of 20:05, 9 June 2019
Coagulation Factor VIII (3cdz)
| |||||||||||
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 https://en.wikipedia.org/wiki/Factor_VIII [11.01.2019]
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 2.16 2.17 2.18 2.19 2.20 Ngo JC, Huang M, Roth DA, Furie BC, Furie B. Crystal structure of human factor VIII: implications for the formation of the factor IXa-factor VIIIa complex. Structure. 2008 Apr;16(4):597-606. PMID:18400180 doi:10.1016/j.str.2008.03.001
- ↑ 3.0 3.1 Antonarakis SE. Molecular genetics of coagulation factor VIII gene and hemophilia A. Thromb Haemost. 1995 Jul;74(1):322-8. PMID:8578479
- ↑ Ragni MV. Mimicking Factor VIII to Manage the Factor VIII–Deficient State. The New England journal of medicine. 2018 Aug; 379(9): 880-882. doi: 10.1056/NEJMe1808789
- ↑ Patek AJ & Taylor FHL. Hemophilia. II. Some properties of a substance obtained from normal human plasma effective in accelerating the coagulation of hemophilic blood. The Journal of clinical investigation. 1937 Jan; 16(1): 113-124. PMID: 16694450 doi: 10.1172/JCI100829
- ↑ Dallman PR & Pool JG. Treatment of hemophilia with factor VIII concentrates. New England Journal of Medicine. 1968 Jan ; 278(4): 199-202. PMID: 5711341 doi: 10.1056/NEJM196801252780406
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 7.6 7.7 7.8 El Khorassani M & Benkirane AN. Le facteur VIII coagulant. Médecine du Maghreb. 1996; 55: 11-13.
- ↑ Ljung RC. Prevention and management of bleeding episodes in children with hemophilia. Pediatric Drugs. 2018 Aug; 1-10. doi https://doi.org/10.1007/s40272-018-0307-z
- ↑ 9.0 9.1 https://www.uniprot.org/uniprot/P00451 [11.01.2019]
- ↑ 10.0 10.1 10.2 http://www.rcsb.org/structure/3CDZ [11.01.2019]
- ↑ 11.0 11.1 11.2 11.3 Toole JJ, Pittman DD, Orr EC, Murtha P, Wasley LC & Kaufman RJ. A large region (approximately equal to 95 kDa) of human factor VIII is dispensable for in vitro procoagulant activity. Proceedings of the National Academy of Sciences. 1986 Aug; 83(16): 5939-5942. PMID: 3016730 doi https://doi.org/10.1073/pnas.83.16.5939
- ↑ 12.0 12.1 12.2 12.3 12.4 12.5 12.6 12.7 12.8 Bihoreau N, Fontaine-Aupart MP, Lehegarat A, Desmadril M, Yon JM. First determination of the secondary structure of purified factor VIII light chain. Biochem J. 1992 Nov; 288 ( Pt 1): 35-40. PMID:1445279 doi: 10.1042/bj2880035
- ↑ 13.0 13.1 13.2 13.3 13.4 Srivastava A, Brewer AK, Mauser‐Bunschoten EP, Key NS, Kitchen S, Llinas A, Ludlam CA, Mahlangu JN, Mulder K, Poon MC & Street A. Guidelines for the management of hemophilia. Haemophilia. 2013 Jan; 19(1): e1-e47. PMID: 22776238 doi: 10.1111/j.1365-2516.2012.02909.x
- ↑ 14.0 14.1 14.2 Konkle BA, Huston H & Fletcher SH. Hemophilia A, Synonym: Factor VIII Deficiency. Gene Rewiews. 2017 Jun. PMID: 20301578
- ↑ White GC, Rosendaal F, Aledort LM, Lusher JM, Rothschild C, Ingerslev J. Definitions in hemophilia, Recommendation of the scientific subcommittee on factor VIII and factor IX of the scientific and standardization committee of the International Society on Thrombosis and Haemostasis. Thromb Haemost. 2001 Mar; 85(3): 560. PMID: 11307831