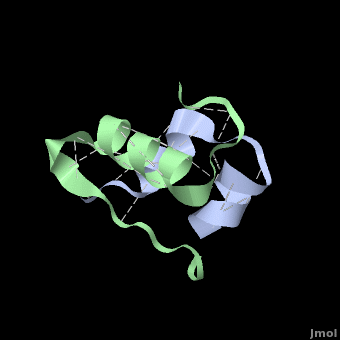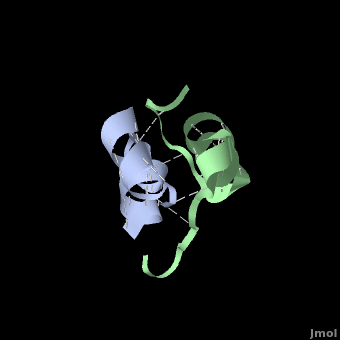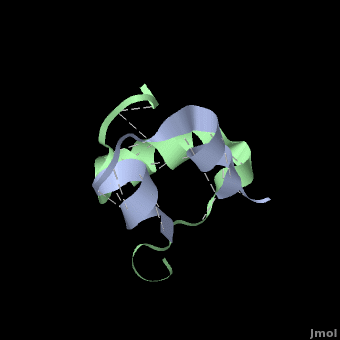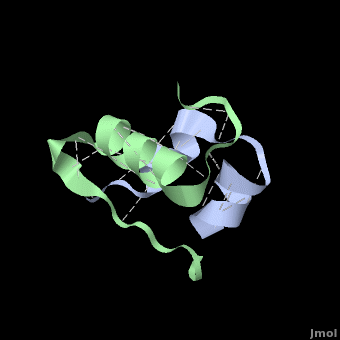Molecular Playground/Insulin
From Proteopedia
| Line 1: | Line 1: | ||
One of the [[CBI Molecules]] being studied in the [http://www.umass.edu/cbi/ University of Massachusetts Amherst Chemistry-Biology Interface Program] at UMass Amherst in the [http://robertsgroup.ecs.umass.edu/ Roberts Research Group] and on display at the [http://www.molecularplayground.org Molecular Playground]. | One of the [[CBI Molecules]] being studied in the [http://www.umass.edu/cbi/ University of Massachusetts Amherst Chemistry-Biology Interface Program] at UMass Amherst in the [http://robertsgroup.ecs.umass.edu/ Roberts Research Group] and on display at the [http://www.molecularplayground.org Molecular Playground]. | ||
| - | + | ||
[[Image:InsulinHexamer.jpg|200px|left]] | [[Image:InsulinHexamer.jpg|200px|left]] | ||
| - | '''Insulin''' is a hormone that controls [[Carbohydrate Metabolism|carbohydrate metabolism]] and storage in the human body<ref>PMID:10927996</ref>. | + | '''Insulin''' is a hormone that controls [[Carbohydrate Metabolism|carbohydrate metabolism]] and storage in the human body<ref>PMID:10927996</ref><ref>DOI:10.1111/dom.13402</ref>. |
| + | |||
| + | ==Function== | ||
| + | |||
| + | The body is able to sense the concentration of glucose in the blood and respond by secreting insulin, which is produced by beta cells in the pancreas. | ||
| + | |||
| + | ==Disease== | ||
| + | Synthesis of human insulin in ''E. coli'' is important to producing insulin for the treatment of type 1 diabetes. It is believed that the hydrophobic sections on the B-chain cause insulin aggregation which initially caused problems in the manufacture and storage of insulin for [[Pharmaceutical_Drugs#Treatments|pharmaceutical use]]. | ||
| + | |||
| + | <StructureSection load='' size='350' side='right' scene='User:Whitney_Stoppel/sandbox1/Human_insulin2/1' caption='Human insulin chain A (grey) and chain B (green), [[3i40]]'> | ||
| + | |||
| + | ==Structure of mature insulin== | ||
| + | Insulin is made up of two pieces called the A- and B-chain, shown in grey and green respectively. These two chains are joined by disulfide bonds, which are shown in yellow. This single piece made up of the A- and B-chains is the active form of the insulin hormone. This is the form that binds the insulin receptor on fat or muscle cells in the body, singling them to take up glucose, or sugar, from the blood and save it for later. | ||
| + | |||
| + | ==Targeting, processing, and storage== | ||
| + | '''Proinsulin''' (Pins) is processed by several proteases in the Golgi apparatus to form insulin which is shorter by 35 amino acids<ref>PMID:15289650</ref>. Shortened B chain insulin analogues are: DPI is a monomeric des-pentapeptide (B26-B30) Ins analogue<ref>PMID:14979729</ref>. DTRI is a monomeric des-tripeptide (B28-B30) Ins analogue. DHPI is for des-heptapeptide (B24-B30) Ins. '''Leginsulin''' (LIns) is a legume Ins. [[Insulin glargine]] is an Ins analogue used for patients with type 1 and 2 diabetes. | ||
| + | |||
| + | Insulin is able to pair-up with itself and form a dimer by forming hydrogen bonds between the ends of two B-chains. These <scene name='User:Whitney_Stoppel/sandbox1/Insulin_dimer/2'>hydrogen bonds</scene> are shown above in white. Then, 3 dimers can come together in the presence of zinc ions and form a hexamer. Insulin is stored in the <scene name='User:Whitney_Stoppel/sandbox1/Insulin_hexamer/4'>hexameric form</scene> in the body. This <scene name='User:Whitney_Stoppel/sandbox1/Insulin_ph7/2'>scene highlights</scene> the hydrophobic (gray) and polar (purple) parts of an insulin monomer at a pH of 7. | ||
| + | </StructureSection> | ||
| + | |||
| + | |||
| + | ==See also== | ||
| + | [[Insulin Strucgture and Function]] | ||
| + | |||
| + | [[Diabetes & Hypoglycemia]] | ||
| + | |||
| + | [[Insulin glargine]] | ||
| - | + | [[Insulin (Hebrew)]] | |
| - | Insulin is able to pair-up with itself and form a dimer by forming hydrogen bonds between the ends of two B-chains. These <scene name='User:Whitney_Stoppel/sandbox1/Insulin_dimer/2'>hydrogen bonds</scene> are shown above in white. Then, 3 dimers can come together in the presence of zinc ions and form a hexamer. Insulin is stored in the <scene name='User:Whitney_Stoppel/sandbox1/Insulin_hexamer/4'>hexameric form</scene> in the body. This <scene name='User:Whitney_Stoppel/sandbox1/Insulin_ph7/2'>scene highlights</scene> the hydrophobic (gray) and polar (purple) parts of an insulin monomer at a pH of 7. It is believed that the hydrophobic sections on the B-chain cause insulin aggregation which initially caused problems in the manufacture and storage of insulin for [[Pharmaceutical_Drugs#Treatments|pharmaceutical use]]. | ||
| - | </StructureSection> For additional details see<br /> | ||
| - | [[Insulin Structure & Function]]<br /> | ||
| - | [[Diabetes & Hypoglycemia]]<br /> | ||
| - | [[Insulin glargine]]<br /> | ||
| - | [[Insulin (Hebrew)]]<br /> | ||
[[Insulin mo-or-sl]] (Hebrew). | [[Insulin mo-or-sl]] (Hebrew). | ||
Revision as of 13:24, 10 July 2019
One of the CBI Molecules being studied in the University of Massachusetts Amherst Chemistry-Biology Interface Program at UMass Amherst in the Roberts Research Group and on display at the Molecular Playground.
Insulin is a hormone that controls carbohydrate metabolism and storage in the human body[1][2].
Contents |
Function
The body is able to sense the concentration of glucose in the blood and respond by secreting insulin, which is produced by beta cells in the pancreas.
Disease
Synthesis of human insulin in E. coli is important to producing insulin for the treatment of type 1 diabetes. It is believed that the hydrophobic sections on the B-chain cause insulin aggregation which initially caused problems in the manufacture and storage of insulin for pharmaceutical use.
| |||||||||||
See also
Insulin Strucgture and Function
Insulin mo-or-sl (Hebrew).
3D structures of Insulin
(Updated on 10-July-2019)
References
- ↑ Sonksen P, Sonksen J. Insulin: understanding its action in health and disease. Br J Anaesth. 2000 Jul;85(1):69-79. PMID:10927996
- ↑ Weiss MA, Lawrence MC. A thing of beauty: Structure and function of insulin's "aromatic triplet". Diabetes Obes Metab. 2018 Sep;20 Suppl 2:51-63. doi: 10.1111/dom.13402. PMID:30230175 doi:http://dx.doi.org/10.1111/dom.13402
- ↑ Davidson HW. (Pro)Insulin processing: a historical perspective. Cell Biochem Biophys. 2004;40(3 Suppl):143-58. PMID:15289650
- ↑ Zakova L, Barth T, Jiracek J, Barthova J, Zorad S. Shortened insulin analogues: marked changes in biological activity resulting from replacement of TyrB26 and N-methylation of peptide bonds in the C-terminus of the B-chain. Biochemistry. 2004 Mar 2;43(8):2323-31. PMID:14979729 doi:http://dx.doi.org/10.1021/bi036001w
Additional Resources
For additional information, see: Diabetes & Hypoglycemia
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Joel L. Sussman, Shelly Livne, Karsten Theis, David Canner, Whitney Stoppel, Alexander Berchansky, Yael Shwartz, Lynmarie K Thompson, Jaime Prilusky