User:Karsten Theis/Insulin
From Proteopedia
(→Structure) |
|||
| Line 93: | Line 93: | ||
===Storage=== | ===Storage=== | ||
| - | Before insulin is secreted, it is able to pair-up with itself and form a dimer by forming hydrogen bonds between the ends of two B-chains, which then combine in threes to form a hexamer (a trimer of dimers to be exact). In crystal structures, insulin occurs in two states, <scene name='82/821037/Rvst/1'>T or R</scene>. In an | + | Before insulin is secreted, it is able to pair-up with itself and form a dimer by forming hydrogen bonds between the ends of two B-chains, which then combine in threes to form a hexamer (a trimer of dimers to be exact). In crystal structures, insulin occurs in two states, <scene name='82/821037/Rvst/1'>T or R</scene>. In an <scene name='82/821037/Rvst/2'>animation</scene> (takes long to load), you can see the conformational change in chain B while maintaining the organisation of the dimer. |
(A) <scene name='82/821037/T6/1'>T6 hexamer (e.g. structure 4INS) </scene>, (B) T3Rf3 hexamer (e.g. 1TRZ) and (C) R6 hexamer (e.g. 1ZNJ). Small changes in the sequence of insulin change how fast hexamers fall apart into monomers. This is used to make insulin preparations that give off low levels of insulin for a longer time, or high levels of insulin for a short time when injected as microcrystals in depots under the skin. | (A) <scene name='82/821037/T6/1'>T6 hexamer (e.g. structure 4INS) </scene>, (B) T3Rf3 hexamer (e.g. 1TRZ) and (C) R6 hexamer (e.g. 1ZNJ). Small changes in the sequence of insulin change how fast hexamers fall apart into monomers. This is used to make insulin preparations that give off low levels of insulin for a longer time, or high levels of insulin for a short time when injected as microcrystals in depots under the skin. | ||
Revision as of 01:34, 14 July 2019
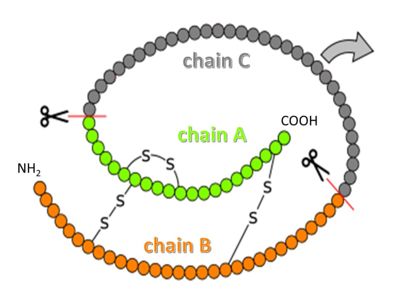
Insulin is a peptide hormone that helps to maintain blood sugar within a healthy range by regulating carbohydrate and lipid metabolism throughout the body[1][2]. It is secreted by specialized cells in the pancreas and acts by binding to insulin receptors on other cells. Insulin in its mature form contains two peptide chains connected by disulfide crosslinks, and occurs either as monomer or as hexamer. Administering insulin in carefully determined doses at the appropriate times is used in managing diabetes, a chronic condition where the body fails to maintain blood sugar levels by itself.
Contents |
Function
Insulin, together with glucagon, regulates blood sugar levels by changing fuel metabolism in all cells [3] on a timescale of minutes and hours. In simple terms, the presence of insulin in the blood signals the well-fed stage, while the presence of glucagon signals the fasting stage. [1]
- Biosynthesis
"The pancreas of a normal adult contains approximately 200 units of insulin, and the average daily secretion of insulin into the circulation in healthy individuals ranges from 30 to 50 units. https://www.britannica.com/science/insulin" 1 IU = 0.0347 mg
foo1[4]
- Secretion and transport
- Receptor interaction
foo3[6]
- Degradation
foo4[7]
Receptor recycling [8]
The body is able to sense the concentration of glucose in the blood and respond by secreting insulin, which is produced by beta cells in the pancreas. Insulin then binds to the insulin receptor, changing its conformation [9]. Depending on cell type and the presence of other signals (glucogon, epinephrine), the cell will modify its metabolism.
Disease and Treatment
In patients with diabetes, insulin signalling is compromised[10].
Type I: Insulin is not produced
Type II: Insulin is produced, but receptor does not trigger signal: [11]
Synthesis of human insulin in E. coli is important to producing insulin for the treatment of type 1 diabetes. It is believed that the hydrophobic sections on the B-chain cause insulin aggregation which initially caused problems in the manufacture and storage of insulin for pharmaceutical use.
In patients with diabetes, insulin signalling is compromised[13]. Synthesis of human insulin in E. coli is important to producing insulin for the treatment of type 1 diabetes. It is believed that the hydrophobic sections on the B-chain cause insulin aggregation which initially caused problems in the manufacture and storage of insulin for pharmaceutical use.
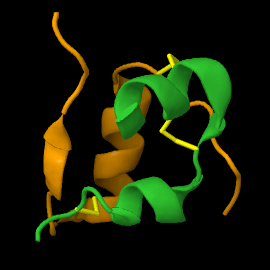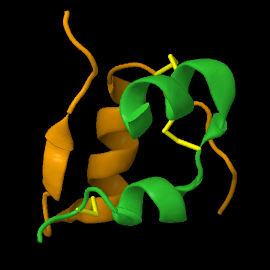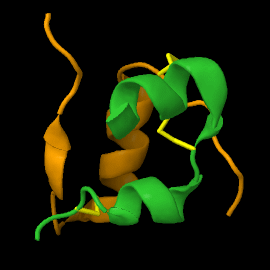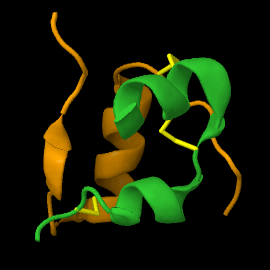
Structure
| |||||||||||
References
- ↑ Sonksen P, Sonksen J. Insulin: understanding its action in health and disease. Br J Anaesth. 2000 Jul;85(1):69-79. PMID:10927996
- ↑ Weiss MA, Lawrence MC. A thing of beauty: Structure and function of insulin's "aromatic triplet". Diabetes Obes Metab. 2018 Sep;20 Suppl 2:51-63. doi: 10.1111/dom.13402. PMID:30230175 doi:http://dx.doi.org/10.1111/dom.13402
- ↑ https://www.yourhormones.info/hormones/insulin/
- ↑ https://www.who.int/biologicals/expert_committee/BS_2143_Human_Recombinant_Insulin_final.pdf
- ↑ "https://www.diabetesselfmanagement.com/diabetes-resources/definitions/portal-vein/"
- ↑ https://pdb101.rcsb.org/motm/182
- ↑ Duckworth WC, Bennett RG, Hamel FG. Insulin degradation: progress and potential. Endocr Rev. 1998 Oct;19(5):608-24. doi: 10.1210/edrv.19.5.0349. PMID:9793760 doi:http://dx.doi.org/10.1210/edrv.19.5.0349
- ↑ Haeusler RA, McGraw TE, Accili D. Biochemical and cellular properties of insulin receptor signalling. Nat Rev Mol Cell Biol. 2018 Jan;19(1):31-44. doi: 10.1038/nrm.2017.89. Epub 2017 , Oct 4. PMID:28974775 doi:http://dx.doi.org/10.1038/nrm.2017.89
- ↑ Gutmann T, Kim KH, Grzybek M, Walz T, Coskun U. Visualization of ligand-induced transmembrane signaling in the full-length human insulin receptor. J Cell Biol. 2018 May 7;217(5):1643-1649. doi: 10.1083/jcb.201711047. Epub 2018, Feb 16. PMID:29453311 doi:http://dx.doi.org/10.1083/jcb.201711047
- ↑ https://www.endotext.org/section/diabetes/
- ↑ Samuel VT, Shulman GI. The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. J Clin Invest. 2016 Jan;126(1):12-22. doi: 10.1172/JCI77812. Epub 2016 Jan 4. PMID:26727229 doi:http://dx.doi.org/10.1172/JCI77812
- ↑ doi: https://dx.doi.org/10.1530/endoabs.56.PL5
- ↑ https://www.endotext.org/section/diabetes/
- ↑ Davidson HW. (Pro)Insulin processing: a historical perspective. Cell Biochem Biophys. 2004;40(3 Suppl):143-58. PMID:15289650