Sandbox reserved 1560
From Proteopedia
(Difference between revisions)
| Line 18: | Line 18: | ||
== Broader Implications == | == Broader Implications == | ||
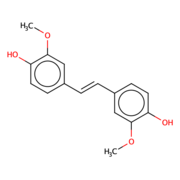
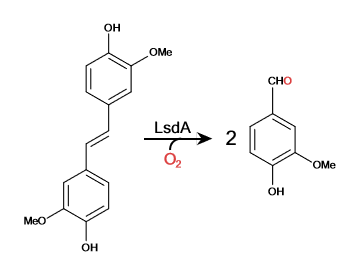
Lignocellulosic biomasses have been used for biofuels, mainly bio-ethanol. The abundant avaiability of the raw material make the use of biomasses ideal. With the rise of carbon emission, biomasses can be used to substitue the need for carbon based fuel, as long as, the biomass is carbon neutral.<ref>Carroll, Andrew; Somerville, Chris (June 2009). "Cellulosic Biofuels". Annual Review of Plant Biology. 60 (1): 165–182. doi:10.1146/annurev.arplant.043008.092125.<ref/> Lignostilbene is the second most abundant raw material. | Lignocellulosic biomasses have been used for biofuels, mainly bio-ethanol. The abundant avaiability of the raw material make the use of biomasses ideal. With the rise of carbon emission, biomasses can be used to substitue the need for carbon based fuel, as long as, the biomass is carbon neutral.<ref>Carroll, Andrew; Somerville, Chris (June 2009). "Cellulosic Biofuels". Annual Review of Plant Biology. 60 (1): 165–182. doi:10.1146/annurev.arplant.043008.092125.<ref/> Lignostilbene is the second most abundant raw material. | ||
| + | |||
== Energy Transformation == | == Energy Transformation == | ||
When Phe 59 is substituted with His and Tyr 101 is substituted with Phe, the k (app/cat) decreases in activity by 15-10 folds. The Lys 134 substitution of Met inhibits the enzyme activity. Phenylazophenol inhibits the LsdA-catalyzed cleavage of lignostilbene with a competitive and uncompetitive inhibition. | When Phe 59 is substituted with His and Tyr 101 is substituted with Phe, the k (app/cat) decreases in activity by 15-10 folds. The Lys 134 substitution of Met inhibits the enzyme activity. Phenylazophenol inhibits the LsdA-catalyzed cleavage of lignostilbene with a competitive and uncompetitive inhibition. | ||
Revision as of 16:25, 2 December 2019
Lignostilbene-α,β-dioxygenase A (LsdA) Catalyzation
| |||||||||||
References
- ↑ Kuatsjah E, Verstraete MM, Kobylarz MJ, Liu AKN, Murphy MEP, Eltis LD. Identification of functionally important residues and structural features in a bacterial lignostilbene dioxygenase. J Biol Chem. 2019 Jul 10. pii: RA119.009428. doi: 10.1074/jbc.RA119.009428. PMID:31292192 doi:http://dx.doi.org/10.1074/jbc.RA119.009428
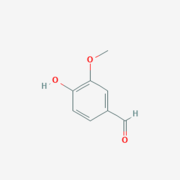
- ↑ https://pubchem.ncbi.nlm.nih.gov/compound/Vanillin
- ↑ Carroll, Andrew; Somerville, Chris (June 2009). "Cellulosic Biofuels". Annual Review of Plant Biology. 60 (1): 165–182. doi:10.1146/annurev.arplant.043008.092125.<ref></ref> Lignostilbene is the second most abundant raw material.
Energy Transformation
When Phe 59 is substituted with His and Tyr 101 is substituted with Phe, the k (app/cat) decreases in activity by 15-10 folds. The Lys 134 substitution of Met inhibits the enzyme activity. Phenylazophenol inhibits the LsdA-catalyzed cleavage of lignostilbene with a competitive and uncompetitive inhibition.
Structural highlights
and .