We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox reserved 1560
From Proteopedia
(Difference between revisions)
| Line 33: | Line 33: | ||
A unique structure of the LsdA is the <scene name='83/830391/Rainbow_7blade_beta_propeller/2'>seven-bladed β-propeller</scene> that is found very commonly in CCOs and helps form a funnel-like active site. A few amino acids that interact with the ligand in the <scene name='83/830391/Binding_site/1'>active site</scene> by hydrogen bonding are His 167 in pink, Glu 135 in red, and Phe 308 in indigo. The <scene name='83/830391/Spacefill_of_lignostilbene/1'>spacefill</scene> of the quaternary structure shows the interaction of atoms within the structure and how they fit within each other. | A unique structure of the LsdA is the <scene name='83/830391/Rainbow_7blade_beta_propeller/2'>seven-bladed β-propeller</scene> that is found very commonly in CCOs and helps form a funnel-like active site. A few amino acids that interact with the ligand in the <scene name='83/830391/Binding_site/1'>active site</scene> by hydrogen bonding are His 167 in pink, Glu 135 in red, and Phe 308 in indigo. The <scene name='83/830391/Spacefill_of_lignostilbene/1'>spacefill</scene> of the quaternary structure shows the interaction of atoms within the structure and how they fit within each other. | ||
The <scene name='83/830391/Hydrophobic/1'>hydrophobic</scene> regions are shown in grey and the polar regions are in magenta. The interactions of hydrophobicity show how the structure interacts with itself as typically hydrophilic regions are found within the structure and hydrophobic regions become the outer most regions of the protein. | The <scene name='83/830391/Hydrophobic/1'>hydrophobic</scene> regions are shown in grey and the polar regions are in magenta. The interactions of hydrophobicity show how the structure interacts with itself as typically hydrophilic regions are found within the structure and hydrophobic regions become the outer most regions of the protein. | ||
| + | |||
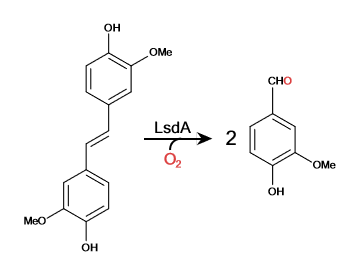
| + | The <scene name='82/823083/Metal_binding_site/1'>metal-binding site</scene> is surrounded by histidines that allow for the Iron ion to be kept in place. The four histidines (HIS 167, HIS 218, HIS 282, and HIS 472) form a tetragonal pyramidal and found in the active site. A cap on the seven-bladed β-propeller helps protect it from the solvent. The ion is delocalized when the hydroxystilbenoid is activated by the enzyme-catalyzed deprotonation of the 4-hydroxy group. It can also form a cation when πelectron density from the scissile double bond redistributes the iron-oxy complex. forming a peroxo-substrate cation intermediate. | ||
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Current revision
| This Sandbox is Reserved from Aug 26 through Dec 12, 2019 for use in the course CHEM 351 Biochemistry taught by Bonnie_Hall at the Grand View University, Des Moines, USA. This reservation includes Sandbox Reserved 1556 through Sandbox Reserved 1575. |
To get started:
More help: Help:Editing |
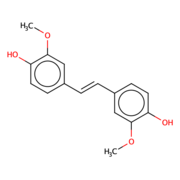
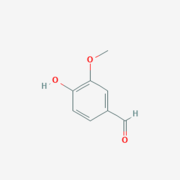
Lignostilbene-α,β-dioxygenase A (LsdA) Catalyzation
| |||||||||||
References
- ↑ Kuatsjah E, Verstraete MM, Kobylarz MJ, Liu AKN, Murphy MEP, Eltis LD. Identification of functionally important residues and structural features in a bacterial lignostilbene dioxygenase. J Biol Chem. 2019 Jul 10. pii: RA119.009428. doi: 10.1074/jbc.RA119.009428. PMID:31292192 doi:http://dx.doi.org/10.1074/jbc.RA119.009428
- ↑ https://pubchem.ncbi.nlm.nih.gov/compound/Vanillin
- ↑ Carroll A, Somerville C. Cellulosic biofuels. Annu Rev Plant Biol. 2009;60:165-82. doi: 10.1146/annurev.arplant.043008.092125. PMID:19014348 doi:http://dx.doi.org/10.1146/annurev.arplant.043008.092125