We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Daniel Mulawa/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 10: | Line 10: | ||
===Overall Structure=== | ===Overall Structure=== | ||
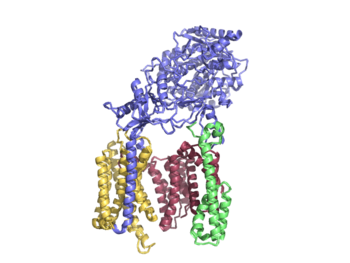
| - | 20 transmembrane components (TMs) | + | Γ-secretase is composed of 20 transmembrane components (TMs) and has 4 subunits: Nicastran, Anterior Pharynx-defective 1, Presenilin, and Presenilin Enhancer 2. These subunits are stabilized by hydrophobic interactions and 4 phosphatidylcholines.These phosphatidylcholines have interfaces between: PS1 and PEN-2, APH-1 and PS1, APH-1 and NCT. |
| - | 4 subunits | + | Nicastrin (NCT) has a large extracellular domain and 1 TM. It is important to substrate recognition and binding. |
| - | Nicastran | + | Presenilin (PS1) serves as the active site of the protease and contains 9 TMs, each varying in length. The site of autocatalytic cleavage is located between TM6 and TM7 in PS1 and major conformational changes take place in this subunit upon substrate binding. |
| + | Anterior pharynx-defective 1 (APH-1) serves as a scaffold for anchoring and supporting the flexible conformational changes of PS1 | ||
| + | Activation of the active site is dependent on the binding of Presenilin enhancer 2 (PEN-2). PEN-2 is also important in maturation of the enzyme. | ||
| + | |||
[[Image:Ribbon structure of whole.png|350 px|right|thumb|Figure 1]] | [[Image:Ribbon structure of whole.png|350 px|right|thumb|Figure 1]] | ||
[http://http://proteopedia.org/wiki/index.php/5a63 5A63 Article] | [http://http://proteopedia.org/wiki/index.php/5a63 5A63 Article] | ||
| Line 30: | Line 33: | ||
===Active Site=== | ===Active Site=== | ||
| + | The active site is located between TM6 and TM7 of the PS1 subunit, which is mainly hydrophilic and disordered. Each of these transmembrane helices has an aspartate residue, Asp257 and Asp385, which are located approximately 10.6 A˚ apart when inactive. | ||
==Relevance== | ==Relevance== | ||
Revision as of 20:41, 24 March 2020
Gamma Secretase
=Human Gamma Secretase=
| |||||||||||
References
1.Bai X, Yan C, Guanghui Yang, et al. 2015. An atomic structure of human γ-secretase. Nature. 525:212-217. 2.Carroll CM, and Li YM. 2016. Physiological and pathological roles of the γ-secretase complex. Brain research bulletin. 126:199-206. 3.Yang G, Zhou R, Shi Y. 2017. Cryo-EM structures of human γ-secretase. Current Opinion in Structural Biology. 46:55–64. 4.Yang G, Zhou R, Zhou Q, et al. 2019. Structural basis of Notch recognition by human γ-secretase. Nature. 565: 192-197. 5.Zhou R, Yang G, Guo X, et al. 2019. Recognition of the amyloid precursor protein by human γ-secretase. Science. 363:1-8.
Student Contributors
Layla Wisser Daniel Mulawa