This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox Reserved 1613
From Proteopedia
(Difference between revisions)
| Line 16: | Line 16: | ||
== Relevance == | == Relevance == | ||
Due to ABCG2 excreting xenobiotics from the cell, they also transport essential cancer drugs out of the cell. By utilizing certain inhibitors (5D3 and MZ29), it is possible to shut down these transporters in order to get cancer drugs to remain in and apoptosis the cell. However, this could have residual effects on the excretory system as ABCG2 would no longer be able to excrete anything from the cell, including those detrimental to the body. | Due to ABCG2 excreting xenobiotics from the cell, they also transport essential cancer drugs out of the cell. By utilizing certain inhibitors (5D3 and MZ29), it is possible to shut down these transporters in order to get cancer drugs to remain in and apoptosis the cell. However, this could have residual effects on the excretory system as ABCG2 would no longer be able to excrete anything from the cell, including those detrimental to the body. | ||
| - | ==Structural | + | == Structural highlights == |
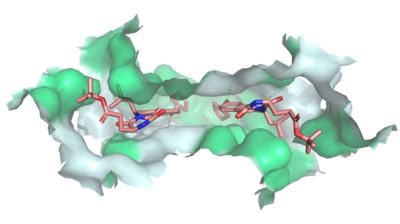
| + | [[Image:Ligand_Interactions_6ffc.png|400 px|right|thumb|Figure 1]] | ||
| + | |||
| + | [https://www.nature.com/articles/s41594-018-0049-1 Nature_Structural_&_Molecular_Biology_Vol_25] | ||
| + | [https://www.nature.com/articles/s41586-018-0680-3] | ||
| + | |||
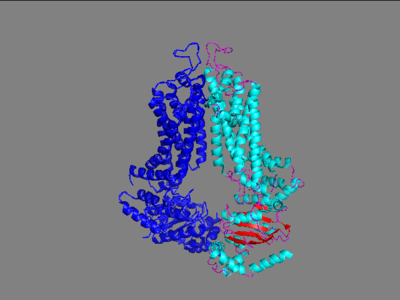
| + | Multidrug Transporter ABCG2 is a <scene name='83/832937/Dimer/1'>dimer</scene> that consists of two cavities seperated by a <scene name='83/832937/Leucine_plug/1'>leucine plug</scene>. Cavity 1 is a binding pocket open to the cytoplasm and the inner leaflet of the plasma membrane. Its shape is suitable to bind flat, hydrophobic and polycyclic substrates. Many of its amino acids residues form hydrophobic interactions with the bound substrate, as shown in green in '''Figure 1'''. Cavity 2 is located above the leucine plug. It is empty until a <scene name='83/832937/Atp_and_mg_bound_to_abcg2/2'>magnesium ion and ATP</scene> are bound to ABCG2. Its <scene name='83/832937/Cysteine_disulfide_bridges/2'>inter- and intra-disulfides</scene> promote the release of the substrate from the cavity into the extracellular space. | ||
| + | |||
</StructureSection> | </StructureSection> | ||
==References== | ==References== | ||
Revision as of 23:56, 30 March 2020
ABCG2 Transporter Protein
| |||||||||||
References
- ↑ Jackson SM, Manolaridis I, Kowal J, Zechner M, Taylor NMI, Bause M, Bauer S, Bartholomaeus R, Bernhardt G, Koenig B, Buschauer A, Stahlberg H, Altmann KH, Locher KP. Structural basis of small-molecule inhibition of human multidrug transporter ABCG2. Nat Struct Mol Biol. 2018 Apr;25(4):333-340. doi: 10.1038/s41594-018-0049-1. Epub, 2018 Apr 2. PMID:29610494 doi:http://dx.doi.org/10.1038/s41594-018-0049-1
- ↑ Manolaridis I, Jackson SM, Taylor NMI, Kowal J, Stahlberg H, Locher KP. Cryo-EM structures of a human ABCG2 mutant trapped in ATP-bound and substrate-bound states. Nature. 2018 Nov;563(7731):426-430. doi: 10.1038/s41586-018-0680-3. Epub 2018 Nov, 7. PMID:30405239 doi:http://dx.doi.org/10.1038/s41586-018-0680-3
- ↑ Taylor NMI, Manolaridis I, Jackson SM, Kowal J, Stahlberg H, Locher KP. Structure of the human multidrug transporter ABCG2. Nature. 2017 Jun 22;546(7659):504-509. doi: 10.1038/nature22345. Epub 2017 May, 29. PMID:28554189 doi:http://dx.doi.org/10.1038/nature22345
Student Contributors
Samuel Sullivan Jaelyn Voyles Shelby Skaggs