We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1613
From Proteopedia
(Difference between revisions)
| Line 14: | Line 14: | ||
== Relevance == | == Relevance == | ||
| - | By utilizing certain binders (<scene name='83/832939/5d3-fab/2'>5D3</scene>), it is able to be stabilized for crystallographic imaging.<ref name="Taylor">PMID:28554189</ref> This has allowed researchers in the past decade to make advances based upon the greater understanding of its structure. Using these advances, inhibitors have been found to stop effluxion by ABCG2. Completely inhibiting this function, however, has residual effects on the excretory system. One such effect is decreased uric acid excretion in both the kidneys and the gut, which causes hyperuricemia. This results in an increased risk of uric acid crystal build-up, known as tophi formation, which causes a type of arthritis known as gout. Balancing the inhibition of ABCG2 will determine how to lessen these effects while continuing to combat cancer resistivity.<ref name="Cleophas">PMID:28461764</ref> | + | By utilizing certain binders (<scene name='83/832939/5d3-fab/2'>5D3</scene>), it is able to be stabilized for crystallographic imaging.<ref name="Taylor">PMID:28554189</ref> This has allowed researchers in the past decade to make advances based upon the greater understanding of its structure. Using these advances, inhibitors have been found to stop effluxion by ABCG2. Completely inhibiting this function, however, has residual effects on the excretory system. One such effect is decreased uric acid excretion in both the kidneys and the gut, which causes [https://en.wikipedia.org/wiki/Hyperuricemia hyperuricemia] . This results in an increased risk of uric acid crystal build-up, known as [https://en.wikipedia.org/wiki/Tophus tophi formation], which causes a type of arthritis known as [https://en.wikipedia.org/wiki/Gout gout] . Balancing the inhibition of ABCG2 will determine how to lessen these effects while continuing to combat cancer resistivity.<ref name="Cleophas">PMID:28461764</ref> |
| - | + | ||
| - | + | ||
| - | + | ||
== Disease == | == Disease == | ||
| - | One of the causes for multidrug resistant cancers is the excretion of cancer drugs out of the cell, thereby decreasing the effective intracellular concentration. ABCG2, also known as the breast cancer resistance protein (BCRP), effluxes multiple chemotherapeutic agents such as mitoxantrone and camptothecin analogies, making the cancerous breast cells resistant to chemotherapy. Competitive inhibitors, such as <scene name='83/832939/Abcg2_bound_to_mz29/3'>MZ29</scene>, that shut down ABCG2 to stop the efflux of cancer drugs in order to combat the resistivity of breast cancer. <ref>[ https://en.wikipedia.org/wiki/ABCG2 "ABCG2 -." Wikipedia, the Free Encyclopedia. Web. 20 Apr. 2020].</ref><ref name=”Jackson”>PMID:29610494</ref> | + | One of the causes for multidrug resistant cancers is the excretion of cancer drugs out of the cell, thereby decreasing the effective intracellular concentration. ABCG2, also known as the breast cancer resistance protein (BCRP), effluxes multiple chemotherapeutic agents such as [https://en.wikipedia.org/wiki/Mitoxantrone mitoxantrone] and [https://en.wikipedia.org/wiki/Camptothecin camptothecin] analogies, making the cancerous breast cells resistant to chemotherapy. Competitive inhibitors, such as <scene name='83/832939/Abcg2_bound_to_mz29/3'>MZ29</scene>, that shut down ABCG2 to stop the efflux of cancer drugs in order to combat the resistivity of breast cancer. <ref>[ https://en.wikipedia.org/wiki/ABCG2 "ABCG2 -." Wikipedia, the Free Encyclopedia. Web. 20 Apr. 2020].</ref><ref name=”Jackson”>PMID:29610494</ref> |
| - | + | ||
| - | + | ||
== Structural highlights == | == Structural highlights == | ||
| Line 28: | Line 23: | ||
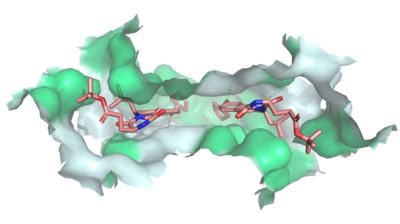
Multidrug Transporter ABCG2 is a <scene name='83/832937/Dimer/1'>dimer</scene> that consists of two cavities seperated by a <scene name='83/832937/Leucine_plug/3'>leucine plug</scene>. Cavity 1 is a binding pocket open to the cytoplasm and the inner leaflet of the plasma membrane. Its shape is suitable to bind flat, hydrophobic and polycyclic substrates. Many of its amino acids residues form hydrophobic interactions with the bound substrate, as shown in green in '''Figure 1'''. Cavity 2 is located above the leucine plug. It is empty until a <scene name='83/832937/Atp_and_mg_bound_to_abcg2/3'>magnesium ion and ATP</scene> are bound to ABCG2. Its <scene name='83/832937/Cysteine_disulfide_bridges/4'>inter- and intra-disulfides</scene> (red is inter- and intra-molecular disulfides, purple is intra-molecular only) promote the release of the substrate from the cavity into the extracellular space. | Multidrug Transporter ABCG2 is a <scene name='83/832937/Dimer/1'>dimer</scene> that consists of two cavities seperated by a <scene name='83/832937/Leucine_plug/3'>leucine plug</scene>. Cavity 1 is a binding pocket open to the cytoplasm and the inner leaflet of the plasma membrane. Its shape is suitable to bind flat, hydrophobic and polycyclic substrates. Many of its amino acids residues form hydrophobic interactions with the bound substrate, as shown in green in '''Figure 1'''. Cavity 2 is located above the leucine plug. It is empty until a <scene name='83/832937/Atp_and_mg_bound_to_abcg2/3'>magnesium ion and ATP</scene> are bound to ABCG2. Its <scene name='83/832937/Cysteine_disulfide_bridges/4'>inter- and intra-disulfides</scene> (red is inter- and intra-molecular disulfides, purple is intra-molecular only) promote the release of the substrate from the cavity into the extracellular space. | ||
| - | <ref name=”Jackson”>PMID:29610494</ref> | + | <ref name=”Jackson”>PMID:29610494</ref> |
<ref name="Manolaridis">PMID:30405239</ref> | <ref name="Manolaridis">PMID:30405239</ref> | ||
Revision as of 03:35, 21 April 2020
ABCG2 Transporter Protein
| |||||||||||
References
- ↑ Fetsch PA, Abati A, Litman T, Morisaki K, Honjo Y, Mittal K, Bates SE. Localization of the ABCG2 mitoxantrone resistance-associated protein in normal tissues. Cancer Lett. 2006 Apr 8;235(1):84-92. doi: 10.1016/j.canlet.2005.04.024. Epub, 2005 Jun 28. PMID:15990223 doi:http://dx.doi.org/10.1016/j.canlet.2005.04.024
- ↑ Taylor NMI, Manolaridis I, Jackson SM, Kowal J, Stahlberg H, Locher KP. Structure of the human multidrug transporter ABCG2. Nature. 2017 Jun 22;546(7659):504-509. doi: 10.1038/nature22345. Epub 2017 May, 29. PMID:28554189 doi:http://dx.doi.org/10.1038/nature22345
- ↑ Cleophas MC, Joosten LA, Stamp LK, Dalbeth N, Woodward OM, Merriman TR. ABCG2 polymorphisms in gout: insights into disease susceptibility and treatment approaches. Pharmgenomics Pers Med. 2017 Apr 20;10:129-142. doi: 10.2147/PGPM.S105854., eCollection 2017. PMID:28461764 doi:http://dx.doi.org/10.2147/PGPM.S105854
- ↑ [ https://en.wikipedia.org/wiki/ABCG2 "ABCG2 -." Wikipedia, the Free Encyclopedia. Web. 20 Apr. 2020].
- ↑ Jackson SM, Manolaridis I, Kowal J, Zechner M, Taylor NMI, Bause M, Bauer S, Bartholomaeus R, Bernhardt G, Koenig B, Buschauer A, Stahlberg H, Altmann KH, Locher KP. Structural basis of small-molecule inhibition of human multidrug transporter ABCG2. Nat Struct Mol Biol. 2018 Apr;25(4):333-340. doi: 10.1038/s41594-018-0049-1. Epub, 2018 Apr 2. PMID:29610494 doi:http://dx.doi.org/10.1038/s41594-018-0049-1
- ↑ Jackson SM, Manolaridis I, Kowal J, Zechner M, Taylor NMI, Bause M, Bauer S, Bartholomaeus R, Bernhardt G, Koenig B, Buschauer A, Stahlberg H, Altmann KH, Locher KP. Structural basis of small-molecule inhibition of human multidrug transporter ABCG2. Nat Struct Mol Biol. 2018 Apr;25(4):333-340. doi: 10.1038/s41594-018-0049-1. Epub, 2018 Apr 2. PMID:29610494 doi:http://dx.doi.org/10.1038/s41594-018-0049-1
- ↑ Manolaridis I, Jackson SM, Taylor NMI, Kowal J, Stahlberg H, Locher KP. Cryo-EM structures of a human ABCG2 mutant trapped in ATP-bound and substrate-bound states. Nature. 2018 Nov;563(7731):426-430. doi: 10.1038/s41586-018-0680-3. Epub 2018 Nov, 7. PMID:30405239 doi:http://dx.doi.org/10.1038/s41586-018-0680-3
Student Contributors
Shelby Skaggs, Samuel Sullivan, Jaelyn Voyles