We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1621
From Proteopedia
(Difference between revisions)
| Line 4: | Line 4: | ||
===Background=== | ===Background=== | ||
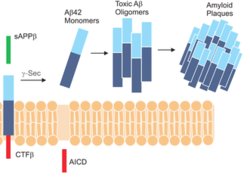
| - | Gamma secretase (GS) is a transmembrane [https://en.wikipedia.org/wiki/Aspartic_protease aspartatic protease] that catalyzes peptide bond hydrolysis of type I [https://en.wikipedia.org/wiki/Integral_membrane_protein integral membrane proteins] such as [https://en.wikipedia.org/wiki/Notch_signaling_pathway Notch], [https://en.wikipedia.org/wiki/Amyloid_precursor_protein amyloid precursor protein (APP)], and various other substrates. GS recognizes and catalyzes the cleavage of its substrate into 3 residue segments.<ref name="Bolduc" /> Initial APP processing by [https://en.wikipedia.org/wiki/Beta-secretase_1 Beta secretase] includes the 48-residue peptide Aβ48 or the 49-residue peptide Aβ49. GS then cleaves these peptides into a variety of peptide fragments separated by 3 residues; Aβ48 is cleaved into Aβ45, Aβ42, and Aβ38; Aβ49 is cleaved into Aβ46, Aβ43, and Aβ40. [https://en.wikipedia.org/wiki/Amyloid_beta Aβ products] are connected to neurological diseases such as [https://en.wikipedia.org/wiki/Alzheimer%27s_disease Alzheimer's disease (AD)], with varying length peptide products showing different disease symptoms. The connection between GS and AD has made GS a popular drug target. Although various inhibitors of GS have been identified, no inhibitors have been clinically approved for treating AD, as GS is also linked to important neurological functions and inhibition of these GS functions leads to dangerous side effects upon inhibition.<ref name="Zhou">PMID:30630874</ref> Recent cryo-electron microscopy (cryo-EM) structures of gamma secretase have showcased the complex four subunit architecture of gamma secretase, the dynamics required for substrate recognition and cleavage, and the differential binding of gamma secretase to the substrates APP and Notch.<ref name="Bai_2015"/> <ref name="Bai"/><ref name="Yang">PMID:30598546</ref><ref name="Zhou"/> | + | Gamma secretase (GS) is a transmembrane [https://en.wikipedia.org/wiki/Aspartic_protease aspartatic protease] that catalyzes peptide bond hydrolysis of type I [https://en.wikipedia.org/wiki/Integral_membrane_protein integral membrane proteins] such as [https://en.wikipedia.org/wiki/Notch_signaling_pathway Notch], [https://en.wikipedia.org/wiki/Amyloid_precursor_protein amyloid precursor protein (APP)], and various other substrates. GS recognizes and catalyzes the cleavage of its substrate into 3 residue segments.<ref name="Bolduc" /> Initial APP processing by [https://en.wikipedia.org/wiki/Beta-secretase_1 Beta secretase] includes the 48-residue peptide Aβ48 or the 49-residue peptide Aβ49. GS then cleaves these peptides into a variety of peptide fragments separated by 3 residues; Aβ48 is cleaved into Aβ45, Aβ42, and Aβ38; Aβ49 is cleaved into Aβ46, Aβ43, and Aβ40. [https://en.wikipedia.org/wiki/Amyloid_beta Aβ products] are connected to neurological diseases such as [https://en.wikipedia.org/wiki/Alzheimer%27s_disease Alzheimer's disease (AD)], with varying length peptide products showing different disease symptoms. The connection between GS and AD has made GS a popular drug target. Although various inhibitors of GS have been identified, no inhibitors have been clinically approved for treating AD, as GS is also linked to important neurological functions and inhibition of these GS functions leads to dangerous side effects upon inhibition.<ref name="Zhou">PMID:30630874</ref> Recent cryo-electron microscopy (cryo-EM) structures of gamma secretase have showcased the complex four subunit architecture of gamma secretase, the dynamics required for substrate recognition and cleavage, and the differential binding of gamma secretase to the substrates APP and Notch.<ref name="Bai_2015"/>,<ref name="Bai"/>,<ref name="Yang">PMID:30598546</ref>,<ref name="Zhou"/> |
===Overall Structure=== | ===Overall Structure=== | ||
Revision as of 13:44, 14 May 2020
Gamma Secretase
References
- ↑ 1.0 1.1 1.2 Bolduc DM, Montagna DR, Seghers MC, Wolfe MS, Selkoe DJ. The amyloid-beta forming tripeptide cleavage mechanism of gamma-secretase. Elife. 2016 Aug 31;5. doi: 10.7554/eLife.17578. PMID:27580372 doi:http://dx.doi.org/10.7554/eLife.17578
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 Zhou R, Yang G, Guo X, Zhou Q, Lei J, Shi Y. Recognition of the amyloid precursor protein by human gamma-secretase. Science. 2019 Feb 15;363(6428). pii: science.aaw0930. doi:, 10.1126/science.aaw0930. Epub 2019 Jan 10. PMID:30630874 doi:http://dx.doi.org/10.1126/science.aaw0930
- ↑ 3.0 3.1 Bai XC, Rajendra E, Yang G, Shi Y, Scheres SH. Sampling the conformational space of the catalytic subunit of human gamma-secretase. Elife. 2015 Dec 1;4. pii: e11182. doi: 10.7554/eLife.11182. PMID:26623517 doi:http://dx.doi.org/10.7554/eLife.11182
- ↑ 4.0 4.1 4.2 4.3 Bai XC, Yan C, Yang G, Lu P, Ma D, Sun L, Zhou R, Scheres SH, Shi Y. An atomic structure of human gamma-secretase. Nature. 2015 Aug 17. doi: 10.1038/nature14892. PMID:26280335 doi:http://dx.doi.org/10.1038/nature14892
- ↑ 5.0 5.1 5.2 5.3 Yang G, Zhou R, Zhou Q, Guo X, Yan C, Ke M, Lei J, Shi Y. Structural basis of Notch recognition by human gamma-secretase. Nature. 2019 Jan;565(7738):192-197. doi: 10.1038/s41586-018-0813-8. Epub 2018 Dec, 31. PMID:30598546 doi:http://dx.doi.org/10.1038/s41586-018-0813-8
- ↑ Bachurin SO, Bovina EV, Ustyugov AA. Drugs in Clinical Trials for Alzheimer's Disease: The Major Trends. Med Res Rev. 2017 Sep;37(5):1186-1225. doi: 10.1002/med.21434. Epub 2017 Jan 13. PMID:28084618 doi:http://dx.doi.org/10.1002/med.21434
- ↑ Kumar D, Ganeshpurkar A, Kumar D, Modi G, Gupta SK, Singh SK. Secretase inhibitors for the treatment of Alzheimer's disease: Long road ahead. Eur J Med Chem. 2018 Mar 25;148:436-452. doi: 10.1016/j.ejmech.2018.02.035. Epub , 2018 Feb 15. PMID:29477076 doi:http://dx.doi.org/10.1016/j.ejmech.2018.02.035
Student Contributors
Daniel Mulawa
Layla Wisser