User:Bruna Oliveira de Almeida/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 2: | Line 2: | ||
<StructureSection load='1stp' size='350' side='right' caption='SARS-Cov-2 RNA polymerase RNA dependent (PDB entry [[1stp]])' scene=''> | <StructureSection load='1stp' size='350' side='right' caption='SARS-Cov-2 RNA polymerase RNA dependent (PDB entry [[1stp]])' scene=''> | ||
| - | |||
== Introduction == | == Introduction == | ||
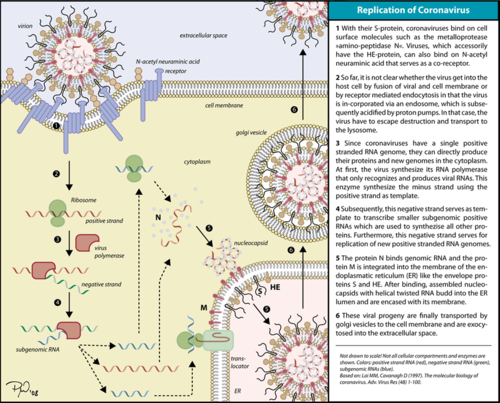
The RNA dependent RNA polymerase (RdRp) of SARS-CoV-2 (also known as nsp12), which is responsible for a major outbreak of the disease called [[Coronavirus Disease 2019 (COVID-19)]], declared pandemic by WHO in 11 march 2020 <ref name="WHO">WHO. COVID-19 situation reports [Internet]. [cited 2020 May 15]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports </ref> is an enzyme that catalyzes the synthesis of RNA of the virus SARS-CoV-2 <ref name="structure1"> PMID: 32277040 </ref>(2). This virus belongs to the betacoronavirus genus<ref name="pneumonia">PMID: 32015507</ref>(Zhou et al. 2020) and has a single stranded positive-sense RNA genome. Therefore, RNA dependent RNA polymerase plays a central role in the virus replication and livecycle. It’s also has been considered a good target for antiviral drugs <ref name="structure1"/>(2). | The RNA dependent RNA polymerase (RdRp) of SARS-CoV-2 (also known as nsp12), which is responsible for a major outbreak of the disease called [[Coronavirus Disease 2019 (COVID-19)]], declared pandemic by WHO in 11 march 2020 <ref name="WHO">WHO. COVID-19 situation reports [Internet]. [cited 2020 May 15]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports </ref> is an enzyme that catalyzes the synthesis of RNA of the virus SARS-CoV-2 <ref name="structure1"> PMID: 32277040 </ref>(2). This virus belongs to the betacoronavirus genus<ref name="pneumonia">PMID: 32015507</ref>(Zhou et al. 2020) and has a single stranded positive-sense RNA genome. Therefore, RNA dependent RNA polymerase plays a central role in the virus replication and livecycle. It’s also has been considered a good target for antiviral drugs <ref name="structure1"/>(2). | ||
| Line 14: | Line 13: | ||
== Structure == | == Structure == | ||
=== Overview === | === Overview === | ||
| - | The RdRp bonded to NSP7 and NSP8 consists of an approximately 160 kDa protein complex (8). The RNA dependent RNA polymerase of SARS-CoV-2 contains 942 amino acid residues while NSP7 has 198 and NSP8 83 residues. (To view the primary and secondary structure of SARS-CoV-2 RdRp and its cofactors visit https://www.rcsb.org/pdb/explore/remediatedSequence.do?structureId=6m71). The structure of RdRp protein of COVID-19 virus contains four domains: a “right hand” RdRp domain (residues S367-F920), a nidovirus-unique N-terminal extension domain (residues D60-R249), which has a nidovirus RdRp-associated nucleotidyltransferase domain (NiRAN) architecture, an interface domain (residues A250-R365) that connects the RdRp domain and NiRAN domain, and a newly identified β-hairpin domain at its N terminus (residues D29-K50) (2). | + | The RdRp bonded to NSP7 and NSP8 consists of an approximately 160 kDa protein complex (8). The RNA dependent RNA polymerase of SARS-CoV-2 contains 942 amino acid residues while NSP7 has 198 and NSP8 83 residues. (To view the primary and secondary structure of SARS-CoV-2 RdRp and its cofactors visit https://www.rcsb.org/pdb/explore/remediatedSequence.do?structureId=6m71). The structure of RdRp protein of COVID-19 virus contains four domains: a “right hand” RdRp domain (residues S367-F920), a nidovirus-unique N-terminal extension domain (residues D60-R249), which has a nidovirus RdRp-associated nucleotidyltransferase domain (NiRAN) architecture, an interface domain (residues A250-R365) that connects the RdRp domain and NiRAN domain, and a newly identified β-hairpin domain at its N terminus (residues D29-K50)<ref name="structure1"/> (2). |
===RdRp domain=== | ===RdRp domain=== | ||
| - | The RdRp domain has a conserved architectures and comprises three subdomains: a fingers subdomain (residues L366-A581 and K621-G679), a palm subdomain (residues T582-P620 and T680-Q815), and a thumb subdomain (residues H816-E920) (2). Its active site consists of the polymerase motifs A, B, C, D, E, F, and G, with important catalytic residues (759-SDD-761) been located in Motif C (2). As in other viral RNA polymerases (9), the template-directed RNA synthesis is mediated by the RdRp domain motifs (2). | + | The RdRp domain has a conserved architectures and comprises three subdomains: a fingers subdomain (residues L366-A581 and K621-G679), a palm subdomain (residues T582-P620 and T680-Q815), and a thumb subdomain (residues H816-E920)<ref name="structure1"/> (2). Its active site consists of the polymerase motifs A, B, C, D, E, F, and G, with important catalytic residues (759-SDD-761) been located in Motif C<ref name="structure1"/> (2). As in other viral RNA polymerases (9), the template-directed RNA synthesis is mediated by the RdRp domain motifs<ref name="structure1"/> (2). |
===NiRAN and β-hairpin domain=== | ===NiRAN and β-hairpin domain=== | ||
| - | The complete SARS-CoV-2 RdRp protein structure obtained by cryo-EM (2,10) allowed to resolve the N-terminal portion of the NiRAN domain as well to identify a N-terminal β-hairpin domain, what was in part unresolved for SARS-CoV RdRp protein (8). It was found that this β-hairpin is inserted in the groove clamped by the NiRAN domain and the palm subdomain and it forms close contacts that help to stabilize the overall structure (2). | + | The complete SARS-CoV-2 RdRp protein structure obtained by cryo-EM (2,10) allowed to resolve the N-terminal portion of the NiRAN domain as well to identify a N-terminal β-hairpin domain, what was in part unresolved for SARS-CoV RdRp protein (8). It was found that this β-hairpin is inserted in the groove clamped by the NiRAN domain and the palm subdomain and it forms close contacts that help to stabilize the overall structure<ref name="structure1"/> (2). |
==An attractive drug target== | ==An attractive drug target== | ||
Revision as of 00:44, 14 June 2020
SARS-CoV-2 RNA-dependent RNA polymerase
| |||||||||||