User:Jordan Scott/Sandbox RNA polII
From Proteopedia
| Line 3: | Line 3: | ||
[[ Image:Label RNA pol II (1).png|150px|right|thumb| RNAP II transcription process.]] | [[ Image:Label RNA pol II (1).png|150px|right|thumb| RNAP II transcription process.]] | ||
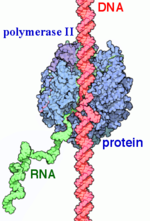
| + | RNA Polymerases (RNAPs) are a group of enzymes that synthesize RNA in a process called transcription. During transcription the polymerase reads the DNA template strand and produces a RNA strand complementary to the template strand. The nascent RNA matches the DNA coding strand. Transcription can be divided into three processes that are discussed below: initiation, elongation and termination. Transcription in eukaryotes requires more distinct proteins for effective control. We see this as prokaryotic organisms have one core polymerase that synthesizes all of their RNA. However, eukaryotes have three distinct RNAPs named RNAP I, II, and III. RNAP I synthesizes rRNA precursors and RNAP III makes tRNA and the 5s rRNA. (A) | ||
| + | |||
| + | |||
| + | RNAP II is responsible for the synthesis of pre-mRNA and snRNs. It is 550 kDa and made of 12 subunits that range from 220-10 kDa. The subunits are highly conserved to the point that mammalian subunits can substitute with yeast subunits are there are little to no defects.(B0) There are two large sub units t and 10 smaller subunits, some of which are shared with RNAPs I and III. While RNAP II is capable of transcription by itself it is non-selective of any particular DNA region. However some mutageneis studies have shown that RNAP II may have some role in selectivity. (A) To properly recognize regions upstream of the gene's transcription start site it requires several general transcription factors that are selective for these regions known as promoters and positions RNAP to accurately begin transcription. (B) These GTF's and other accessriy proteins called SRBs are neccesary for accurate transcription and togetther with teh RNAP II core enzyme form the holoenzyme. | ||
'''RNA polymerase II''' (RNAP II) is an enzyme that transcribes protein-encoding genes, and it therefore is responsible for the synthesis of mRNA. There are three RNA polymerase enzymes found in eukaryotic nuclei but RNAP II is the most studied. RNAP II is a 550 kDa multi-protein complex that includes 12 subunits. Several transcription factors are used to bind promoters upstream of the start site and are necessary for joining RNAP II and DNA. Bound RNAP II transcribes DNA into a strand of messenger RNA (mRNA). mRNA is a single stranded RNA molecule that is complementary to the template strand of DNA and matches the coding strand. In prokaryotes the transcript is ready for translation, but in eukaryotes post transcriptional modifications are required. The mRNA stand transports genetic information from DNA to the ribosome,where the code will be translated into an amino acid acid sequence to form proteins. | '''RNA polymerase II''' (RNAP II) is an enzyme that transcribes protein-encoding genes, and it therefore is responsible for the synthesis of mRNA. There are three RNA polymerase enzymes found in eukaryotic nuclei but RNAP II is the most studied. RNAP II is a 550 kDa multi-protein complex that includes 12 subunits. Several transcription factors are used to bind promoters upstream of the start site and are necessary for joining RNAP II and DNA. Bound RNAP II transcribes DNA into a strand of messenger RNA (mRNA). mRNA is a single stranded RNA molecule that is complementary to the template strand of DNA and matches the coding strand. In prokaryotes the transcript is ready for translation, but in eukaryotes post transcriptional modifications are required. The mRNA stand transports genetic information from DNA to the ribosome,where the code will be translated into an amino acid acid sequence to form proteins. | ||
| + | |||
| + | ===Subunits=== | ||
| + | The 12 subunits that make up | ||
===History=== | ===History=== | ||
The Discovery and Isolation of RNA Polymerase by Jerard Hurwitz | The Discovery and Isolation of RNA Polymerase by Jerard Hurwitz | ||
| - | ===Learning Objectives=== | ||
| - | Understand the structure of RNAP II | ||
| - | Look at the processes of Initiation, Elongation, and Termination | ||
| - | Gain a molecular and mechanistic view of these prcoesses | ||
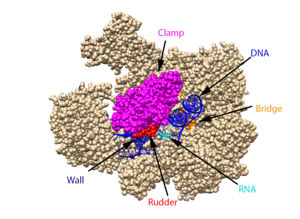
== Structural Components == | == Structural Components == | ||
| Line 44: | Line 47: | ||
Process of PIC formation: | Process of PIC formation: | ||
| + | |||
| + | https://www.rcsb.org/structure/5VVS Use this for scene making | ||
| + | |||
Source: https://www.pnas.org/content/94/1/151. | Source: https://www.pnas.org/content/94/1/151. | ||
| Line 54: | Line 60: | ||
4. <scene name='82/824648/Tfiie/4'>TFIIE</scene> and <scene name='82/824648/Tfiih/3'>TFIIH</scene> are sequentially recruited which completes the <scene name='82/824648/Pic/3'>PIC</scene>. | 4. <scene name='82/824648/Tfiie/4'>TFIIE</scene> and <scene name='82/824648/Tfiih/3'>TFIIH</scene> are sequentially recruited which completes the <scene name='82/824648/Pic/3'>PIC</scene>. | ||
| - | 5.<scene name='82/824648/Tfiia/3'>TFIIA</scene> is a co-activator that helps regulate PIC assembly. It serves as an enhancer and stabilizes the early complexes. It allows for inhibitory effect to be reduced and activators to be more effective. | + | 5.<scene name='82/824648/Tfiia/3'>TFIIA</scene> is a co-activator that helps regulate PIC assembly. It was initially thought to be essential for activity. It serves as an enhancer and stabilizes the early complexes. It allows for inhibitory effect to be reduced and activators to be more effective. |
| Line 63: | Line 69: | ||
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
| + | (A)Young, Richard A. (2003-11-28). "RNA Polymerase II". Annual Review of Biochemistry. 60 (1): 689–715. doi:10.1146/annurev.bi.60.070191.003353. PMID 1883205. | ||
| + | |||
| + | (B) https://www.jbc.org/content/273/43/27757 RNA Polymerase II Holoenzymes and Subcomplexes | ||
| + | |||
| + | (C) RNA polymerase II transcription initiation: A structural view | ||
| + | |||
| + | (D)The Discovery and Isolation of RNA Polymerase by Jerard Hurwitz | ||
Bushnell, D. A.; Westover, K. D.; Davis, R. E.; Kornberg, R. D. Structural Basis of Transcription: An RNA Polymerase II-TFIIB Cocrystal at 4.5 Angstroms. Science. 2004, 303, 983-988 | Bushnell, D. A.; Westover, K. D.; Davis, R. E.; Kornberg, R. D. Structural Basis of Transcription: An RNA Polymerase II-TFIIB Cocrystal at 4.5 Angstroms. Science. 2004, 303, 983-988 | ||
Revision as of 21:36, 26 September 2020
| |||||||||||
References
(A)Young, Richard A. (2003-11-28). "RNA Polymerase II". Annual Review of Biochemistry. 60 (1): 689–715. doi:10.1146/annurev.bi.60.070191.003353. PMID 1883205.
(B) https://www.jbc.org/content/273/43/27757 RNA Polymerase II Holoenzymes and Subcomplexes
(C) RNA polymerase II transcription initiation: A structural view
(D)The Discovery and Isolation of RNA Polymerase by Jerard Hurwitz Bushnell, D. A.; Westover, K. D.; Davis, R. E.; Kornberg, R. D. Structural Basis of Transcription: An RNA Polymerase II-TFIIB Cocrystal at 4.5 Angstroms. Science. 2004, 303, 983-988
Brueckner, F. and Cramer, P. Structural Basis of Transcription Inhibition by -amanitin and Implications for RNA Polymerase II Translocation. Nature Structure and Molecular Biology. 2008, 15, 811-818.
Cramer, P.; Bushnell, D. A.; Kornberg, R. D. Structural Basis of Transcription: RNA Polymerase II at 2.8 Ångstrom Resolution. Science. 2001, 292, 1863-1876
Evans, D. A.; Fitch, D. M.; Smith, T. E.; Cee, V. J. Application of Complex Aldol Reactions to the Total Synthesis of Phorboxazole B. J. Am. Chem. Soc. 2000, 122, 10033-10046.
Gnatt, A. L.; Cramer, P; Fu, J.; Bushnell, D. A.; and Kornberg, R. D. Structural Basis of Transcription: An RNA Polymerase II Elongation Complex at 3.3 Å Resolution. Science. 2001, 292, 1876-1882 1i6h
Hahn, S. Structure and Mechanism of the RNA Polymerase II Transcription Machinery. Nature Structure and Molecular Biology. 2004, 11, 394-403.
He, Yuan, et al. Near-atomic resolution visualization of human transcription promoter opening. Nature 533.7603. 2016.
Nudler, E. RNA Polymerase Active Center: The Molecular Engine of Transcription. Annu. Rev. Biochem. 2009, 78, 335-361.
Orphanides, George, Thierry Lagrange, and Danny Reinberg. The general transcription factors of RNA polymerase II. Genes & development 10.21. 1996. 2657-2683
Shah, N. et. al. Tyrosine-1 of RNA Polymerase II CTD Controls Global Termination of Gene Transcription in Mammals. Molecular Cell. 2018, 69, 48-61.
Uzman, A.; Voet, D. Student companion Fundamentals of biochemistry: life at the molecular level, 4th ed., Donald Voet, Judith G. Voet, Charlotte W. Pratt; John Wiley & amp; Sons, 2012.
Xu, J.; Lahiri, I.; Wang, W.; Wier, A.; Cianfrocco, M. A.; Chong, J.; Hare, A. A.; Dervan, P. B.; DiMaio, F.; Leschziner, A. E.; Wang, D. Structural Basis for the Initiation of Eukaryotic Transcription-coupled DNA Repair. Nature. 2017. 551, 653-657 5vvr
Xin, L.; Bushnell, D. A.; and Kornburg, R. D. RNA Polymerase II Transcription: Structure and Mechanism. Biochemica et Biophysica Acta. 2013, 1829, 2-8.
Yan, C., Dodd, T., He, Y., Tainer, J. A., Tsutakawa, S. E., & Ivanov, I. (2019). Transcription preinitiation complex structure and dynamics provide insight into genetic diseases. Nature Structural and Molecular Biology, 26(6), 397-406.
Alpha-aminitin chemical structure image courtesy of https://en.wikipedia.org/wiki/Alpha-Amanitin#/media/File:Alpha-amanitin_structure.png
Notes
From structural components:
Structural overview: [PDB: 5VVR: with highlighted sections mentioned below]
Bridge: Depicted: [PDB: 1I6H: 810-845.a]
Wall: Depicted: [PDB: 1R5U: 853-919.b; 933-972.b]
Clamp: Depicted: [PDB: 1R5U: 3-345.a; 1395-1435.a; 1158-1124.b]
Rudder: Depicted: [PDB: 5VVR: 306-321.a]
Content Donators
This page was created as a final project for the Advanced Biochemistry course at Wabash College during the Fall of 2019. This page was reviewed by Dr. Wally Novak of Wabash College.