From Proteopedia
(Difference between revisions)
proteopedia linkproteopedia link
|
|
| Line 6: |
Line 6: |
| | | | |
| | | | |
| - | RNAP II is responsible for the synthesis of pre-mRNA and snRNs. It is 550 kDa and made of 12 subunits that range from 220-10 kDa. The subunits are highly conserved to the point that mammalian subunits can substitute with yeast subunits are there are little to no defects.(B0) There are two large sub units t and 10 smaller subunits, some of which are shared with RNAPs I and III. While RNAP II is capable of transcription by itself it is non-selective of any particular DNA region. However some mutageneis studies have shown that RNAP II may have some role in selectivity. (A) To properly recognize regions upstream of the gene's transcription start site it requires several general transcription factors that are selective for these regions known as promoters and positions RNAP to accurately begin transcription. (B) These GTF's and other accessriy proteins called SRBs are neccesary for accurate transcription and togetther with teh RNAP II core enzyme form the holoenzyme. | + | RNAP II is responsible for the synthesis of pre-mRNA and snRNs. It is 550 kDa and made of 12 subunits that range from 220-10 kDa. The subunits are highly conserved to the point that mammalian subunits can substitute with yeast subunits are there are little to no defects.(B0) There are two large sub units t and 10 smaller subunits, some of which are shared with RNAPs I and III. While RNAP II is capable of transcription by itself it is non-selective of any particular DNA region. However some mutageneis studies have shown that RNAP II may have some role in selectivity. (A) To properly recognize regions upstream of the gene's transcription start site it requires several general transcription factors that are selective for these regions known as promoters and positions RNAP to accurately begin transcription. (B) These GTF's and other accessory proteins called SRBs are necessary for accurate transcription and together with thw RNAP II core enzyme form the RNAP holoenzyme. |
| | | | |
| | '''RNA polymerase II''' (RNAP II) is an enzyme that transcribes protein-encoding genes, and it therefore is responsible for the synthesis of mRNA. There are three RNA polymerase enzymes found in eukaryotic nuclei but RNAP II is the most studied. RNAP II is a 550 kDa multi-protein complex that includes 12 subunits. Several transcription factors are used to bind promoters upstream of the start site and are necessary for joining RNAP II and DNA. Bound RNAP II transcribes DNA into a strand of messenger RNA (mRNA). mRNA is a single stranded RNA molecule that is complementary to the template strand of DNA and matches the coding strand. In prokaryotes the transcript is ready for translation, but in eukaryotes post transcriptional modifications are required. The mRNA stand transports genetic information from DNA to the ribosome,where the code will be translated into an amino acid acid sequence to form proteins. | | '''RNA polymerase II''' (RNAP II) is an enzyme that transcribes protein-encoding genes, and it therefore is responsible for the synthesis of mRNA. There are three RNA polymerase enzymes found in eukaryotic nuclei but RNAP II is the most studied. RNAP II is a 550 kDa multi-protein complex that includes 12 subunits. Several transcription factors are used to bind promoters upstream of the start site and are necessary for joining RNAP II and DNA. Bound RNAP II transcribes DNA into a strand of messenger RNA (mRNA). mRNA is a single stranded RNA molecule that is complementary to the template strand of DNA and matches the coding strand. In prokaryotes the transcript is ready for translation, but in eukaryotes post transcriptional modifications are required. The mRNA stand transports genetic information from DNA to the ribosome,where the code will be translated into an amino acid acid sequence to form proteins. |
| Line 16: |
Line 16: |
| | The Discovery and Isolation of RNA Polymerase by Jerard Hurwitz | | The Discovery and Isolation of RNA Polymerase by Jerard Hurwitz |
| | | | |
| - | RNA Polymerase was first isolated y Jerard Hurwitz in 1960. Prior to this, there was research in the synthesis of RNA. One enzyme that was initally thought to synthesize RNA, known as polynucleotide phosphorylase, was isolated but it was discovered to be involved in the degradation of RNA. | + | RNA Polymerase was first discovered and isolated by Jerard Hurwitz in 1960. Prior to this, there was research in the synthesis of RNA. One enzyme known as polynucleotide phosphorylase was first isolated. It was initially thought to synthesize RNA but it was later discovered to degrade RNA. This spurred Hurwitz to search for RNAP using E.coli extracts. In 1960 he showed reproducible RNA synthesis using his extracts and DNA. He published his findings along with three other labs who had also independently worked with RNAP. |
| | + | |
| | + | After this discovery, Hurwitz, along with John J. Furth, purified the enzyme from the E.coli extracts. The purified enzyme catalyzed RNA in the presence of rNTPs, DNA, and magnesium or manganese ions. |
| | + | |
| | == Structural Components == | | == Structural Components == |
| | | | |
| Line 63: |
Line 66: |
| | | | |
| | | | |
| - | Once the <scene name='82/824648/Pic/3'>PIC</scene> is formed, <scene name='82/824648/Rnap_ii/1'>RNAP II</scene> initiates RNA synthesis and produces a short transcript. When RNAP II becomes phosphorylated, it releases some of the GTFs from the complex and moves away from the promoter. TFIID stays bound to the promoter and can reinitiate transcription and hlep with successive transcription. The transcription factors are replaced by a new six-protein complex call the Elongator. TFIIF and TFIIH both remain associated with RNAP II during elongation. | + | Once the <scene name='82/824648/Pic/3'>PIC</scene> is formed, <scene name='82/824648/Rnap_ii/1'>RNAP II</scene> initiates RNA synthesis and produces a short transcript. When RNAP II becomes phosphorylated, it releases some of the GTFs from the complex and moves away from the promoter. TFIID stays bound to the promoter and can reinitiate transcription and help with successive transcription. The transcription factors are replaced by a new six-protein complex call the Elongator. TFIIF and TFIIH both remain associated with RNAP II during elongation. |
| | | | |
| | | | |
Revision as of 18:01, 27 September 2020
|
Introduction
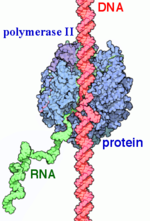 RNAP II transcription process. RNA Polymerases (RNAPs) are a group of enzymes that synthesize RNA in a process called transcription. During transcription the polymerase reads the DNA template strand and produces a RNA strand complementary to the template strand. The nascent RNA matches the DNA coding strand. Transcription can be divided into three processes that are discussed below: initiation, elongation and termination. Transcription in eukaryotes requires more distinct proteins for effective control. We see this as prokaryotic organisms have one core polymerase that synthesizes all of their RNA. However, eukaryotes have three distinct RNAPs named RNAP I, II, and III. RNAP I synthesizes rRNA precursors and RNAP III makes tRNA and the 5s rRNA. (A)
RNAP II is responsible for the synthesis of pre-mRNA and snRNs. It is 550 kDa and made of 12 subunits that range from 220-10 kDa. The subunits are highly conserved to the point that mammalian subunits can substitute with yeast subunits are there are little to no defects.(B0) There are two large sub units t and 10 smaller subunits, some of which are shared with RNAPs I and III. While RNAP II is capable of transcription by itself it is non-selective of any particular DNA region. However some mutageneis studies have shown that RNAP II may have some role in selectivity. (A) To properly recognize regions upstream of the gene's transcription start site it requires several general transcription factors that are selective for these regions known as promoters and positions RNAP to accurately begin transcription. (B) These GTF's and other accessory proteins called SRBs are necessary for accurate transcription and together with thw RNAP II core enzyme form the RNAP holoenzyme.
RNA polymerase II (RNAP II) is an enzyme that transcribes protein-encoding genes, and it therefore is responsible for the synthesis of mRNA. There are three RNA polymerase enzymes found in eukaryotic nuclei but RNAP II is the most studied. RNAP II is a 550 kDa multi-protein complex that includes 12 subunits. Several transcription factors are used to bind promoters upstream of the start site and are necessary for joining RNAP II and DNA. Bound RNAP II transcribes DNA into a strand of messenger RNA (mRNA). mRNA is a single stranded RNA molecule that is complementary to the template strand of DNA and matches the coding strand. In prokaryotes the transcript is ready for translation, but in eukaryotes post transcriptional modifications are required. The mRNA stand transports genetic information from DNA to the ribosome,where the code will be translated into an amino acid acid sequence to form proteins.
Subunits
The 12 subunits that make up
History
The Discovery and Isolation of RNA Polymerase by Jerard Hurwitz
RNA Polymerase was first discovered and isolated by Jerard Hurwitz in 1960. Prior to this, there was research in the synthesis of RNA. One enzyme known as polynucleotide phosphorylase was first isolated. It was initially thought to synthesize RNA but it was later discovered to degrade RNA. This spurred Hurwitz to search for RNAP using E.coli extracts. In 1960 he showed reproducible RNA synthesis using his extracts and DNA. He published his findings along with three other labs who had also independently worked with RNAP.
After this discovery, Hurwitz, along with John J. Furth, purified the enzyme from the E.coli extracts. The purified enzyme catalyzed RNA in the presence of rNTPs, DNA, and magnesium or manganese ions.
Structural Components
This section will briefly discuss the chief structural components involved in the mechanism.
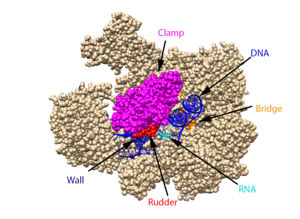 The clamp (magenta), wall (navy blue), rudder (red), bridge (orange), RNA (light blue), and DNA (blue) are depicted. See below for PDB's and residue numbers. To begin, the swings to trap the DNA in the cleft. Further along, the sends the DNA template through the cleft in approximately a 90° turn. Both the clamp and wall are parts of the Rpb2 subunit. Further along in the process, the separates the newly synthesized RNA strand from the DNA template. The DNA reforms into a double helix as it leaves RNA pol II.
Other components of RNA pol II include the following:
The jaw is the opening through which DNA enters. The funnel is what the NTP’s travel through to be incorporated into the growing RNA strand, and the pore is the end of the funnel. The is an Rpb1 segment that translocates the DNA-RNA complex at the end of each cycle of catalysis. is located within the active site and functions as the catalyst.
Transcription
Transcription can largely be divided into three sections: initiation, elongation, and termination. In the process of initiation, RNAP II recruits several general transcription factors (GTFs) to bind to the promoter region of the DNA, and this eventually forms the preinitiation complex (PIC). The DNA enters RNAP II through the clamp, and then it is unwound, creating a transcription bubble. With the DNA unwound, the active site of RNAP II catalyzes the synthesis of the first few RNA bonds. Once the carboxy-terminal domain (CTD) becomes phosphorylated, the clamp undergoes a conformation change to effectively trap the DNA, and a few of the GTFs dissociate, which changes complex to the Elongator complex.
After initiation, the process of elongation begins with the entry of NTPs. These NTPs largely enter through the funnel, and this tends to be a slow process because the funnel is only 12 Å in diameter, meaning that only one NTP can go through at a time. Once they have passed through the funnel, the NTPs enter which contains an Mg ion that is bound by three aspartate residues at positions D739, D741, and D743. If the NTP is complementary to the DNA strand, it is loaded onto the insertion site next to the RNA and held in place by the bridge. While in this insertion site, the metal ion and aspartate residues catalyze the reaction that forms a phosphodiester bond between the 3’ end of the RNA and the 5’ end of the NTP. This step then leads into a translocation step. To make room for the next NTP, there is a Brownian ratchet mechanism in which the nearby trigger loop undergoes a conformation change that causes the bridge to move to the next transition site. During the bridge’s transition, the DNA-RNA hybrid is partially help in place by the -amanitin. Once the bridge is in the new initiation site, the trigger loop returns to its original conformation, allowing the process to begin again.
In the termination stage, the CTD becomes dephosphorylated, which acts as a signal to dissociate the elongation complex. Once this signal is received, the new mRNA is released from RNP II through the rudder. Before being released, its 3’ end is polyadenylated. The DNA is brought back together at the other end of the transcription bubble, returning it to its original double-stranded form. Finally, RNAP II and the remaining GTFs dissociate from the DNA.
α-Amanitin
α-Amanitin is a bicyclic octapeptide that adheres tightly with RNAP II, which blocks the elongation steps. α-amanitin binds in the funnel and interacts with the bridge helix and adjacent Rpb1, but it does not inhibit the RNA pol II’s interaction with NTP. Instead, α-amanitin likely challenges the bridge’s conformational change that is necessary for the purposed RNAP translocation step. α-Amanitin, found in the poisonous mushroom death cap, leads to death after several days. This time frame aligns with the rate at which mRNA’s and proteins turnover.
 The chemical structure of α-amanitin.
General Transcription Factors
In both eukaryotes and prokaryotes, the basic mechanism for initiating transcription is the same: protein factors selectively bind to promoter regions on DNA. Prokaryotes use sigma factors while eukaryotes use a complex of 6 general initiation factors(GIFs). The combination of all the transcription factors bound to the DNA promoter region, in complex with RNAP II, is known as the preinitiation complex. This complex is necessary for an accurate initiation. The formation of the PIC occurs in an ordered pathway, beginning with the TATA box which is a promoter region on DNA at position -27.
Process of PIC formation:
https://www.rcsb.org/structure/5VVS Use this for scene making
Source: https://www.pnas.org/content/94/1/151.
1. is highly conserved among eukaryotes. It recognizes and binds the TATA box. This is facilitated by a subunit named the TATA-binding protein (TBP). This subunit also causes slight deformations in the helix which may be important for further binding of the PIC units.
2. is the second to join the PIC. It is thought to be responsible for stabilizing the TBP/DNA complex and insuring proper directionality . If the orientation is wring unfavorable interactions form. Mutagenesis studies also suggest that it works as a bridge between TFIID and pol II.
3. binds directly to RNAP II and escorts it to the promoter while TFIIB helps the complex bind correctly.
4. and are sequentially recruited which completes the .
5. is a co-activator that helps regulate PIC assembly. It was initially thought to be essential for activity. It serves as an enhancer and stabilizes the early complexes. It allows for inhibitory effect to be reduced and activators to be more effective.
Once the is formed, initiates RNA synthesis and produces a short transcript. When RNAP II becomes phosphorylated, it releases some of the GTFs from the complex and moves away from the promoter. TFIID stays bound to the promoter and can reinitiate transcription and help with successive transcription. The transcription factors are replaced by a new six-protein complex call the Elongator. TFIIF and TFIIH both remain associated with RNAP II during elongation.
|
References
(A)Young, Richard A. (2003-11-28). "RNA Polymerase II". Annual Review of Biochemistry. 60 (1): 689–715. doi:10.1146/annurev.bi.60.070191.003353. PMID 1883205.
(B) https://www.jbc.org/content/273/43/27757 RNA Polymerase II Holoenzymes and Subcomplexes
(C) RNA polymerase II transcription initiation: A structural view
(D)The Discovery and Isolation of RNA Polymerase by Jerard Hurwitz
(E)Multiple forms of DNA-dependent RNA polymerase in eukaryotic organisms
Bushnell, D. A.; Westover, K. D.; Davis, R. E.; Kornberg, R. D. Structural Basis of Transcription: An RNA Polymerase II-TFIIB Cocrystal at 4.5 Angstroms. Science. 2004, 303, 983-988
Brueckner, F. and Cramer, P. Structural Basis of Transcription Inhibition by -amanitin and Implications for RNA Polymerase II Translocation. Nature Structure and Molecular Biology. 2008, 15, 811-818.
Cramer, P.; Bushnell, D. A.; Kornberg, R. D. Structural Basis of Transcription: RNA Polymerase II at 2.8 Ångstrom Resolution. Science. 2001, 292, 1863-1876
Evans, D. A.; Fitch, D. M.; Smith, T. E.; Cee, V. J. Application of Complex Aldol Reactions to the Total Synthesis of Phorboxazole B. J. Am. Chem. Soc. 2000, 122, 10033-10046.
Gnatt, A. L.; Cramer, P; Fu, J.; Bushnell, D. A.; and Kornberg, R. D. Structural Basis of Transcription: An RNA Polymerase II Elongation Complex at 3.3 Å Resolution. Science. 2001, 292, 1876-1882 1i6h
Hahn, S. Structure and Mechanism of the RNA Polymerase II Transcription Machinery. Nature Structure and Molecular Biology. 2004, 11, 394-403.
He, Yuan, et al. Near-atomic resolution visualization of human transcription promoter opening. Nature 533.7603. 2016.
Nudler, E. RNA Polymerase Active Center: The Molecular Engine of Transcription. Annu. Rev. Biochem. 2009, 78, 335-361.
Orphanides, George, Thierry Lagrange, and Danny Reinberg. The general transcription factors of RNA polymerase II. Genes & development 10.21. 1996. 2657-2683
Shah, N. et. al. Tyrosine-1 of RNA Polymerase II CTD Controls Global Termination of Gene Transcription in Mammals. Molecular Cell. 2018, 69, 48-61.
Uzman, A.; Voet, D. Student companion Fundamentals of biochemistry: life at the molecular level, 4th ed., Donald Voet, Judith G. Voet, Charlotte W. Pratt; John Wiley & amp; Sons, 2012.
Xu, J.; Lahiri, I.; Wang, W.; Wier, A.; Cianfrocco, M. A.; Chong, J.; Hare, A. A.; Dervan, P. B.; DiMaio, F.; Leschziner, A. E.; Wang, D. Structural Basis for the Initiation of Eukaryotic Transcription-coupled DNA Repair. Nature. 2017. 551, 653-657 5vvr
Xin, L.; Bushnell, D. A.; and Kornburg, R. D. RNA Polymerase II Transcription: Structure and Mechanism. Biochemica et Biophysica Acta. 2013, 1829, 2-8.
Yan, C., Dodd, T., He, Y., Tainer, J. A., Tsutakawa, S. E., & Ivanov, I. (2019). Transcription preinitiation complex structure and dynamics provide insight into genetic diseases. Nature Structural and Molecular Biology, 26(6), 397-406.
Alpha-aminitin chemical structure image courtesy of https://en.wikipedia.org/wiki/Alpha-Amanitin#/media/File:Alpha-amanitin_structure.png
Notes
From structural components:
Structural overview: [PDB: 5VVR: with highlighted sections mentioned below]
Bridge: Depicted: [PDB: 1I6H: 810-845.a]
Wall: Depicted: [PDB: 1R5U: 853-919.b; 933-972.b]
Clamp: Depicted: [PDB: 1R5U: 3-345.a; 1395-1435.a; 1158-1124.b]
Rudder: Depicted: [PDB: 5VVR: 306-321.a]
Content Donators
This page was created as a final project for the Advanced Biochemistry course at Wabash College during the Fall of 2019. This page was reviewed by Dr. Wally Novak of Wabash College.



