User:Neal Hayhurst/RNA Polymerase II/Sandbox 1
From Proteopedia
| Line 78: | Line 78: | ||
After a short transcript is synthesized during initiation, the process of elongation is stimulated by a conformational transition<ref name="VVP">Voet, D., Voet, J. G., & Pratt, C. W. (2013). Transcription and RNA Processing. In Fundamentals of biochemistry: life at the molecular level (pp. 933–942). Wiley.</ref>. To achieve the conformation conducive for elongation, the <scene name='86/862212/Tfiib_finger_domain/3'>TFIIB</scene> finger domain is displaced away from the active site to make room for the nascent RNA transcript and the CTD of the <scene name='86/862212/Rpb1_subunit/1'>Rpb1 subunit</scene> is phosphorylated<ref name="VVP"/>. Phosphorylation releases some initiation factors--some of these GTFs are left on the promoter region of the template to recruit another RNAPII<ref name="VVP"/>. <scene name='86/862212/Elongator_complex/1'>Elongator complex</scene> replaces the GTFs on the phosphorylated CTD of Rpb1 to accelerate transcription<ref name="VVP"/>. | After a short transcript is synthesized during initiation, the process of elongation is stimulated by a conformational transition<ref name="VVP">Voet, D., Voet, J. G., & Pratt, C. W. (2013). Transcription and RNA Processing. In Fundamentals of biochemistry: life at the molecular level (pp. 933–942). Wiley.</ref>. To achieve the conformation conducive for elongation, the <scene name='86/862212/Tfiib_finger_domain/3'>TFIIB</scene> finger domain is displaced away from the active site to make room for the nascent RNA transcript and the CTD of the <scene name='86/862212/Rpb1_subunit/1'>Rpb1 subunit</scene> is phosphorylated<ref name="VVP"/>. Phosphorylation releases some initiation factors--some of these GTFs are left on the promoter region of the template to recruit another RNAPII<ref name="VVP"/>. <scene name='86/862212/Elongator_complex/1'>Elongator complex</scene> replaces the GTFs on the phosphorylated CTD of Rpb1 to accelerate transcription<ref name="VVP"/>. | ||
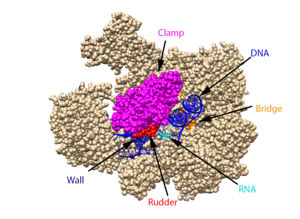
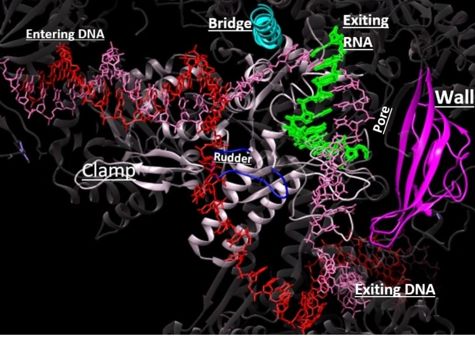
| - | DNA unwinds 3 nucleotides ahead of the active site (contained in Rpb1)<ref name="VVP"/>. Past the active site, the <scene name='86/862212/Wall_domain/1'>wall domain</scene> of Rpb2 redirects the template strand at about a 90o angle out of the cleft, orienting the template base in the active site towards the active site floor to align with the incoming NTP<ref name="VVP"/>. NTPs enter the active site on the floor side through a 12Å funnel called the pore, large enough for only one NTP at a time<ref name="VVP"/>. Once they have passed through the funnel, the NTPs enter <scene name='86/860989/Active_site_wn_test/1'>active site</scene> which is coordinated by three magnesium ions | + | DNA unwinds 3 nucleotides ahead of the active site (contained in Rpb1)<ref name="VVP"/>. Past the active site, the <scene name='86/862212/Wall_domain/1'>wall domain</scene> of Rpb2 redirects the template strand at about a 90o angle out of the cleft, orienting the template base in the active site towards the active site floor to align with the incoming NTP<ref name="VVP"/>. NTPs enter the active site on the floor side through a 12Å funnel called the pore, large enough for only one NTP at a time<ref name="VVP"/>. Once they have passed through the funnel, the NTPs enter <scene name='86/860989/Active_site_wn_test/1'>active site</scene> which is coordinated by three magnesium ions<ref name="Wang">DOI: 10.1016/j.cell.2006.11.023</ref>. |
| - | The <scene name='86/862212/Trigger_loop_and_bridge_helix/1'>trigger loop and bridge helix</scene> domains of Rpb1 are found adjacent to the active site and have been implicated in RNAPII NTP selectivity, catalysis, and translocation | + | The <scene name='86/862212/Trigger_loop_and_bridge_helix/1'>trigger loop and bridge helix</scene> domains of Rpb1 are found adjacent to the active site and have been implicated in RNAPII NTP selectivity, catalysis, and translocation<ref name="Wang" />. The trigger loop oscillates between positions near downstream DNA and near the active site<ref name="Wang" />. When a correctly paired NTP enters the A site, the trigger loop swings under the NTP, closing off the active site and allowing phosphodiester bond formation between the 3' end of the nascent RNA and the 5' end of the NTP<ref name="Wang" />. The trigger loop is stabilized by extensive interactions with the bridge helix, the NTP ribose, base, and phosphate groups, and other active site residues<ref name="Wang" />. The extensive trigger loop contacts slightly unwind and bend the bridge helix, a conformational change thought to play an important role in translocation<ref name="Wang" />. <scene name='86/862212/Trigger_loop/1'>HIS1085</scene> of the trigger loop is thought to catalyze phosphodiester bond formation<ref name="Wang" />. Release of a pyrophosphate group after bond formation destabilizes interactions with His1085, releasing the trigger loop from the active site and allowing movement of the DNA-RNA hybrid helix and entrance of a new NTP<ref name="Wang" />. Becasue the trigger loop maintains the bridge helix conformation, its release allows the bridge helix to relax and such movement likely contributes to RNAPII translocation<ref name="Wang" />. |
====Kinetics==== | ====Kinetics==== | ||
Revision as of 01:44, 1 October 2020
| |||||||||||
References
- ↑ 1.0 1.1 1.2 Young RA. RNA polymerase II. Annu Rev Biochem. 1991;60:689-715. doi: 10.1146/annurev.bi.60.070191.003353. PMID:1883205 doi:http://dx.doi.org/10.1146/annurev.bi.60.070191.003353
- ↑ 2.0 2.1 Myer VE, Young RA. RNA polymerase II holoenzymes and subcomplexes. J Biol Chem. 1998 Oct 23;273(43):27757-60. doi: 10.1074/jbc.273.43.27757. PMID:9774381 doi:http://dx.doi.org/10.1074/jbc.273.43.27757
- ↑ 3.0 3.1 3.2 Sobennikova MV, Shematorova EK, Shpakovskii GV. [C-terminal domain (CTD) of the subunit Rpb1 of nuclear RNA polymerase II and its role in the transcription cycle]. Mol Biol (Mosk). 2007 May-Jun;41(3):433-49. PMID:17685222
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 RNA polymerase II transcription initiation: A structural view D. B. Nikolov, S. K. Burley Proceedings of the National Academy of Sciences Jan 1997, 94 (1) 15-22; DOI: 10.1073/pnas.94.1.15
- ↑ Hurwitz J. The discovery of RNA polymerase. J Biol Chem. 2005 Dec 30;280(52):42477-85. doi: 10.1074/jbc.X500006200. Epub 2005, Oct 17. PMID:16230341 doi:http://dx.doi.org/10.1074/jbc.X500006200
- ↑ doi: https://dx.doi.org/10.1038/nrm1796
- ↑ 7.0 7.1 7.2 7.3 Orphanides, George, Thierry Lagrange, and Danny Reinberg. The general transcription factors of RNA polymerase II. Genes & development 10.21. 1996. 2657-2683
- ↑ 8.0 8.1 Xin, L.; Bushnell, D. A.; and Kornburg, R. D. RNA Polymerase II Transcription: Structure and Mechanism. Biochemica et Biophysica Acta. 2013, 1829, 2-8.
- ↑ Chang-Hui Shen; Diagnostic Molecular Biology, 2019
- ↑ He, Yuan, et al. Near-atomic resolution visualization of human transcription promoter opening. Nature 533.7603. 2016.
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 11.6 11.7 11.8 Hahn, S. Structure and Mechanism of the RNA Polymerase II Transcription Machinery. Nature Structure and Molecular Biology. 2004, 11, 394-403.
- ↑ 12.0 12.1 Eick, D, Geyer, M.The RNA Polymerase II Carboxy-Terminal Domain (CTD) Code. Chemical Reviews, 2013, 113 (11), 8456-8490 DOI: 10.1021/cr400071f
- ↑ Wang W, Carey M, Gralla JD. Polymerase II Promoter Activation: Closed Complex Formation and ATP-Driven Start Site Opening. Science. 1992;255:450–453.
- ↑ 14.0 14.1 14.2 14.3 14.4 14.5 14.6 14.7 14.8 14.9 Voet, D., Voet, J. G., & Pratt, C. W. (2013). Transcription and RNA Processing. In Fundamentals of biochemistry: life at the molecular level (pp. 933–942). Wiley.
- ↑ Nudler, E. RNA Polymerase Active Center: The Molecular Engine of Transcription. Annu. Rev. Biochem. 2009, 78, 335-361.
- ↑ Svetlov, V., & Nudler, E. (2013). Basic mechanism of transcription by RNA polymerase II. Biochimica et biophysica acta, 1829(1), 20–28. https://doi.org/10.1016/j.bbagrm.2012.08.009
- ↑ 17.0 17.1 17.2 17.3 17.4 17.5 17.6 17.7 17.8 Wang D, Bushnell DA, Westover KD, Kaplan CD, Kornberg RD. Structural basis of transcription: role of the trigger loop in substrate specificity and catalysis. Cell. 2006 Dec 1;127(5):941-54. PMID:17129781 doi:http://dx.doi.org/10.1016/j.cell.2006.11.023
- ↑ Bar-Nahum G, Epshtein V, Ruckenstein AE, Rafikov R, Mustaev A, Nudler E. A ratchet mechanism of transcription elongation and its control. Cell. 2005 Jan 28;120(2):183-93. doi: 10.1016/j.cell.2004.11.045. PMID:15680325 doi:http://dx.doi.org/10.1016/j.cell.2004.11.045
Bushnell, D. A.; Westover, K. D.; Davis, R. E.; Kornberg, R. D. Structural Basis of Transcription: An RNA Polymerase II-TFIIB Cocrystal at 4.5 Angstroms. Science. 2004, 303, 983-988
Brueckner, F. and Cramer, P. Structural Basis of Transcription Inhibition by -amanitin and Implications for RNA Polymerase II Translocation. Nature Structure and Molecular Biology. 2008, 15, 811-818.
Cramer, P.; Bushnell, D. A.; Kornberg, R. D. Structural Basis of Transcription: RNA Polymerase II at 2.8 Ångstrom Resolution. Science. 2001, 292, 1863-1876
Evans, D. A.; Fitch, D. M.; Smith, T. E.; Cee, V. J. Application of Complex Aldol Reactions to the Total Synthesis of Phorboxazole B. J. Am. Chem. Soc. 2000, 122, 10033-10046.
Gnatt, A. L.; Cramer, P; Fu, J.; Bushnell, D. A.; and Kornberg, R. D. Structural Basis of Transcription: An RNA Polymerase II Elongation Complex at 3.3 Å Resolution. Science. 2001, 292, 1876-1882 1i6h
Hahn, S. Structure and Mechanism of the RNA Polymerase II Transcription Machinery. Nature Structure and Molecular Biology. 2004, 11, 394-403.
He, Yuan, et al. Near-atomic resolution visualization of human transcription promoter opening. Nature 533.7603. 2016.
Nudler, E. RNA Polymerase Active Center: The Molecular Engine of Transcription. Annu. Rev. Biochem. 2009, 78, 335-361.
Orphanides, George, Thierry Lagrange, and Danny Reinberg. The general transcription factors of RNA polymerase II. Genes & development 10.21. 1996. 2657-2683
Shah, N. et. al. Tyrosine-1 of RNA Polymerase II CTD Controls Global Termination of Gene Transcription in Mammals. Molecular Cell. 2018, 69, 48-61.
Uzman, A.; Voet, D. Student companion Fundamentals of biochemistry: life at the molecular level, 4th ed., Donald Voet, Judith G. Voet, Charlotte W. Pratt; John Wiley & amp; Sons, 2012.
Xu, J.; Lahiri, I.; Wang, W.; Wier, A.; Cianfrocco, M. A.; Chong, J.; Hare, A. A.; Dervan, P. B.; DiMaio, F.; Leschziner, A. E.; Wang, D. Structural Basis for the Initiation of Eukaryotic Transcription-coupled DNA Repair. Nature. 2017. 551, 653-657 5vvr
Xin, L.; Bushnell, D. A.; and Kornburg, R. D. RNA Polymerase II Transcription: Structure and Mechanism. Biochemica et Biophysica Acta. 2013, 1829, 2-8.
Yan, C., Dodd, T., He, Y., Tainer, J. A., Tsutakawa, S. E., & Ivanov, I. (2019). Transcription preinitiation complex structure and dynamics provide insight into genetic diseases. Nature Structural and Molecular Biology, 26(6), 397-406.
Eick, D, Geyer, M.The RNA Polymerase II Carboxy-Terminal Domain (CTD) Code. Chemical Reviews, 2013, 113 (11), 8456-8490 DOI: 10.1021/cr400071f
Chang-Hui Shen; Diagnostic Molecular Biology, 2019
Alpha-aminitin chemical structure image courtesy of https://en.wikipedia.org/wiki/Alpha-Amanitin#/media/File:Alpha-amanitin_structure.png
Notes
From structural components:
Structural overview: [PDB: 5VVR: with highlighted sections mentioned below]
Bridge: Depicted: [PDB: 1I6H: 810-845.a]
Wall: Depicted: [PDB: 1R5U: 853-919.b; 933-972.b]
Clamp: Depicted: [PDB: 1R5U: 3-345.a; 1395-1435.a; 1158-1124.b]
Rudder: Depicted: [PDB: 5VVR: 306-321.a]
Content Donators
This page was created as a final project for the Advanced Biochemistry course at Wabash College during the Fall of 2019 and Fall of 2020. This page was reviewed by Dr. Wally Novak of Wabash College.