This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox Reserved 1652
From Proteopedia
(Difference between revisions)
| Line 7: | Line 7: | ||
[https://en.wikipedia.org/wiki/TRPV1 TRPV1] (Vanilloid Transient Receptor Potential Type 1) is a non-selective ion channel which, in response to a stimulus, induces an incoming current of cations, primarily calcium and sodium, which causes depolarization of the cell. It is part of the [https://en.wikipedia.org/wiki/Transient_receptor_potential_channel TRP] (Transient Receptor Potential) superfamily and is the first in a subfamily of vanilloid-sensitive TRP channels / channels: TRPVs. | [https://en.wikipedia.org/wiki/TRPV1 TRPV1] (Vanilloid Transient Receptor Potential Type 1) is a non-selective ion channel which, in response to a stimulus, induces an incoming current of cations, primarily calcium and sodium, which causes depolarization of the cell. It is part of the [https://en.wikipedia.org/wiki/Transient_receptor_potential_channel TRP] (Transient Receptor Potential) superfamily and is the first in a subfamily of vanilloid-sensitive TRP channels / channels: TRPVs. | ||
| - | This receptor is expressed by sensory neurons of the dorsal and trigeminal spinal ganglia.TRPV1 is implicated in [https://en.wikipedia.org/wiki/Nociception nociception], its activation by heat or by chemical substances leads to a painful sensation.<ref | + | This receptor is expressed by sensory neurons of the dorsal and trigeminal spinal ganglia.TRPV1 is implicated in [https://en.wikipedia.org/wiki/Nociception nociception], its activation by heat or by chemical substances leads to a painful sensation.<ref name="TRPV1">Wikipedia contributors. (2020b, décembre 21). TRPV1. Wikipedia. https://en.wikipedia.org/wiki/TRPV1 (Consulté le: déc. 28, 2020). [En ligne].</ref> |
== Structure of TRPV1 == | == Structure of TRPV1 == | ||
| Line 17: | Line 17: | ||
The N-terminal region has 6 repeats of [https://en.wikipedia.org/wiki/Ankyrin ankyrin].<ref>« Structure of the TRPV1 ion channel determined by electron cryo-microscopy | Nature ».https://www.nature.com/articles/nature12822#Fig3 (consulté le déc. 28, 2020)</ref><ref>G. Smutzer et R. K. Devassy, « Integrating TRPV1 Receptor Function with Capsaicin Psychophysics », Advances in Pharmacological Sciences, janv. 14, 2016</ref> | The N-terminal region has 6 repeats of [https://en.wikipedia.org/wiki/Ankyrin ankyrin].<ref>« Structure of the TRPV1 ion channel determined by electron cryo-microscopy | Nature ».https://www.nature.com/articles/nature12822#Fig3 (consulté le déc. 28, 2020)</ref><ref>G. Smutzer et R. K. Devassy, « Integrating TRPV1 Receptor Function with Capsaicin Psychophysics », Advances in Pharmacological Sciences, janv. 14, 2016</ref> | ||
| - | The transmembrane region is composed of '''six transmembrane a helices''' (S1-S6). S1,S2 and S3 helices contain aromatic side chain (S1 : Y441,Y444,Y555 S2: F488 S3 : F516).<ref>« Structure of the TRPV1 ion channel determined by electron cryo-microscopy | Nature ». https://www.nature.com/articles/nature12822#Fig3 (consulté le déc. 28, 2020)</ref>. A small hydrophobic domain beetween S5 and S6 with a re-entrant loop constitutes the pore allowing the passage of ions through the TRPV1 receptor.<ref | + | The transmembrane region is composed of '''six transmembrane a helices''' (S1-S6). S1,S2 and S3 helices contain aromatic side chain (S1 : Y441,Y444,Y555 S2: F488 S3 : F516).<ref>« Structure of the TRPV1 ion channel determined by electron cryo-microscopy | Nature ». https://www.nature.com/articles/nature12822#Fig3 (consulté le déc. 28, 2020)</ref>. A small hydrophobic domain beetween S5 and S6 with a re-entrant loop constitutes the pore allowing the passage of ions through the TRPV1 receptor.<ref name="TRPV1"/> |
'''Threonin''' residu (T550) and '''tyrosin''' residu (Y511) located on the fifth and the third transmembrane helices are very conserved. Threonin 550 and tyrosin 511 are implicated in TRPV1 activation by [https://en.wikipedia.org/wiki/Vanilloids vanilloids] and in pain sensation.<ref>R. Kumar, A. Hazan, A. Basu, N. Zalcman, H. Matzner, et A. Priel, « Tyrosine Residue in the TRPV1 Vanilloid Binding Pocket Regulates Deactivation Kinetics », J. Biol. Chem., vol. 291, no 26, p. 13855‑13863, juin 2016, doi: 10.1074/jbc.M116.726372.</ref> | '''Threonin''' residu (T550) and '''tyrosin''' residu (Y511) located on the fifth and the third transmembrane helices are very conserved. Threonin 550 and tyrosin 511 are implicated in TRPV1 activation by [https://en.wikipedia.org/wiki/Vanilloids vanilloids] and in pain sensation.<ref>R. Kumar, A. Hazan, A. Basu, N. Zalcman, H. Matzner, et A. Priel, « Tyrosine Residue in the TRPV1 Vanilloid Binding Pocket Regulates Deactivation Kinetics », J. Biol. Chem., vol. 291, no 26, p. 13855‑13863, juin 2016, doi: 10.1074/jbc.M116.726372.</ref> | ||
| Line 38: | Line 38: | ||
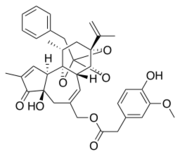
The capsaicin cycle binds via hydrogen bounds to amino acids on the S3 helix (Y511, S513), on the S4-S5 linker (E571) and on the S6 helix (T671). The amid group of capsaicin binds the S4 helix (T551).<ref>G. Smutzer et R. K. Devassy, « Integrating TRPV1 Receptor Function with Capsaicin Psychophysics », Advances in Pharmacological Sciences, janv. 14, 2016.</ref> | The capsaicin cycle binds via hydrogen bounds to amino acids on the S3 helix (Y511, S513), on the S4-S5 linker (E571) and on the S6 helix (T671). The amid group of capsaicin binds the S4 helix (T551).<ref>G. Smutzer et R. K. Devassy, « Integrating TRPV1 Receptor Function with Capsaicin Psychophysics », Advances in Pharmacological Sciences, janv. 14, 2016.</ref> | ||
| - | Capsaicin maintains TRPV1 in an open state by «pull and contact» interactions. A conformational change wave spread over the whole pore.<ref>F. Yang et al., « The conformational wave in capsaicin activation of transient receptor potential vanilloid 1 ion channel », Nat. Commun., vol. 9, no 1, Art. no 1, juill. 2018, doi: 10.1038/s41467-018-05339-6.</ref>. This leads to the massive enter of Ca2+ and Na+ in the cytoplasm of the nerve fiber and to the depolarization of the nerve fiber. When depolarization reach a theshold value it triggers the generation of an [https://en.wikipedia.org/wiki/Action_potential action potential] causing a painful sensation.<ref | + | Capsaicin maintains TRPV1 in an open state by «pull and contact» interactions. A conformational change wave spread over the whole pore.<ref>F. Yang et al., « The conformational wave in capsaicin activation of transient receptor potential vanilloid 1 ion channel », Nat. Commun., vol. 9, no 1, Art. no 1, juill. 2018, doi: 10.1038/s41467-018-05339-6.</ref>. This leads to the massive enter of Ca2+ and Na+ in the cytoplasm of the nerve fiber and to the depolarization of the nerve fiber. When depolarization reach a theshold value it triggers the generation of an [https://en.wikipedia.org/wiki/Action_potential action potential] causing a painful sensation.<ref name="TRPV1"/> |
====Resiniferatoxin (RTX)==== | ====Resiniferatoxin (RTX)==== | ||
Revision as of 21:25, 7 January 2021
| This Sandbox is Reserved from 26/11/2020, through 26/11/2021 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1643 through Sandbox Reserved 1664. |
To get started:
More help: Help:Editing |
The Transient Receptor Potential cation channel subfamily V member 1 TRPV1
| |||||||||||
References
- ↑ 1.0 1.1 1.2 Wikipedia contributors. (2020b, décembre 21). TRPV1. Wikipedia. https://en.wikipedia.org/wiki/TRPV1 (Consulté le: déc. 28, 2020). [En ligne].
- ↑ « Structure of the TRPV1 ion channel determined by electron cryo-microscopy | Nature ». https://www.nature.com/articles/nature12822#Fig3 (consulté le déc. 28, 2020)
- ↑ T. Rosenbaum et S. A. Simon, « TRPV1 Receptors and Signal Transduction », in TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades, W. B. Liedtke et S. Heller, Éd. Boca Raton (FL): CRC Press/Taylor & Francis, 2007
- ↑ « Structure of the TRPV1 ion channel determined by electron cryo-microscopy | Nature ». https://www.nature.com/articles/nature12822#Fig3 (consulté le déc. 28, 2020)
- ↑ « Structure of the TRPV1 ion channel determined by electron cryo-microscopy | Nature ».https://www.nature.com/articles/nature12822#Fig3 (consulté le déc. 28, 2020)
- ↑ G. Smutzer et R. K. Devassy, « Integrating TRPV1 Receptor Function with Capsaicin Psychophysics », Advances in Pharmacological Sciences, janv. 14, 2016
- ↑ « Structure of the TRPV1 ion channel determined by electron cryo-microscopy | Nature ». https://www.nature.com/articles/nature12822#Fig3 (consulté le déc. 28, 2020)
- ↑ R. Kumar, A. Hazan, A. Basu, N. Zalcman, H. Matzner, et A. Priel, « Tyrosine Residue in the TRPV1 Vanilloid Binding Pocket Regulates Deactivation Kinetics », J. Biol. Chem., vol. 291, no 26, p. 13855‑13863, juin 2016, doi: 10.1074/jbc.M116.726372.
- ↑ G. Smutzer et R. K. Devassy, « Integrating TRPV1 Receptor Function with Capsaicin Psychophysics », Advances in Pharmacological Sciences, janv. 14, 2016.
- ↑ « Structure of the TRPV1 ion channel determined by electron cryo-microscopy | Nature ». https://www.nature.com/articles/nature12822#Fig3 (consulté le déc. 28, 2020)
- ↑ X. Yao, H.-Y. Kwan, et Y. Huang, « Regulation of TRP Channels by Phosphorylation », Neurosignals, vol. 14, no 6, p. 273‑280, 2005, doi: 10.1159/000093042
- ↑ F. Yang et J. Zheng, « Understand spiciness: mechanism of TRPV1 channel activation by capsaicin », Protein Cell, vol. 8, no 3, p. 169‑177, mars 2017, doi: 10.1007/s13238-016-0353-7.
- ↑ F. Yang et al., « Structural mechanism underlying capsaicin binding and activation of the TRPV1 ion channel », Nat. Chem. Biol., vol. 11, no 7, Art. no 7, juill. 2015, doi: 10.1038/nchembio.1835.
- ↑ G. Smutzer et R. K. Devassy, « Integrating TRPV1 Receptor Function with Capsaicin Psychophysics », Advances in Pharmacological Sciences, janv. 14, 2016.
- ↑ F. Yang et al., « The conformational wave in capsaicin activation of transient receptor potential vanilloid 1 ion channel », Nat. Commun., vol. 9, no 1, Art. no 1, juill. 2018, doi: 10.1038/s41467-018-05339-6.
- ↑ K. Elokely et al., « Understanding TRPV1 activation by ligands: Insights from the binding modes of capsaicin and resiniferatoxin », Proc. Natl. Acad. Sci., vol. 113, no 2, p. E137‑E145, janv. 2016, doi:10.1073/pnas.1517288113.
- ↑ K. W. Ho, N. J. Ward, et D. J. Calkins, « TRPV1: a stress response protein in the central nervous system », Am. J. Neurodegener. Dis., vol. 1, no 1, p. 1‑14, avr. 2012.
- ↑ G. Smutzer et R. K. Devassy, « Integrating TRPV1 Receptor Function with Capsaicin Psychophysics », Advances in Pharmacological Sciences, janv. 14, 2016.
- ↑ K. W. Ho, N. J. Ward, et D. J. Calkins, « TRPV1: a stress response protein in the central nervous system », Am. J. Neurodegener. Dis., vol. 1, no 1, p. 1‑14, avr. 2012.
- ↑ G. Bhave et al., « Protein kinase C phosphorylation sensitizes but does not activate the capsaicin receptor transient receptor potential vanilloid 1 (TRPV1) », Proc. Natl. Acad. Sci., vol. 100, no 21, p. 12480‑12485, oct. 2003, doi: 10.1073/pnas.2032100100.
- ↑ A. Danigo, L. Magy, et C. Demiot, « TRPV1 dans les neuropathies douloureuses - Des modèles animaux aux perspectives thérapeutiques », médecine/sciences, vol. 29, no 6‑7, Art. no 6‑7, juin 2013, doi: 10.1051/medsci/2013296012.
- ↑ A. Danigo, L. Magy, et C. Demiot, « TRPV1 dans les neuropathies douloureuses - Des modèles animaux aux perspectives thérapeutiques », médecine/sciences, vol. 29, no 6‑7, Art. no 6‑7, juin 2013, doi: 10.1051/medsci/2013296012.