We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1656
From Proteopedia
(Difference between revisions)
| Line 34: | Line 34: | ||
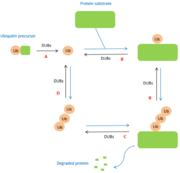
It is the '''catalytic domain''' which defines the family of the DUB. Indeed, DUBs belonging to the family of cysteine proteases have a catalytic site composed of two or three amino acids (dyads or triads). When the catalytic site is active, it may contain cysteine, histidine, aspartate or asparagine residues. In the case of metalloproteases, the active site is composed of a zinc ion and amino acids such as histidine, aspartate and serine. <ref>https://authors.library.caltech.edu/261/1/AMBpb04.pdf</ref> | It is the '''catalytic domain''' which defines the family of the DUB. Indeed, DUBs belonging to the family of cysteine proteases have a catalytic site composed of two or three amino acids (dyads or triads). When the catalytic site is active, it may contain cysteine, histidine, aspartate or asparagine residues. In the case of metalloproteases, the active site is composed of a zinc ion and amino acids such as histidine, aspartate and serine. <ref>https://authors.library.caltech.edu/261/1/AMBpb04.pdf</ref> | ||
| - | The studied structure shows both <scene name='86/868189/Catalytic_site_polyubiquitine/2'>the catalytic site (in orange) and polyubiquitine in purple.</scene> | + | The studied structure shows both <scene name='86/868189/Catalytic_site_polyubiquitine/2'>the catalytic site (in orange) and polyubiquitine (in purple).</scene> |
Residues present in the catalytic site of DUBs are often in a '''non-functional orientation''' when the substrate is absent. Thus, when the substrate binds to the catalytic site of the enzyme, the site undergoes rearrangement and takes on a functional conformation. <ref>PMID:16537382</ref> The substrate opens and closes to allow the entry of the protein to be deubiquitinased. | Residues present in the catalytic site of DUBs are often in a '''non-functional orientation''' when the substrate is absent. Thus, when the substrate binds to the catalytic site of the enzyme, the site undergoes rearrangement and takes on a functional conformation. <ref>PMID:16537382</ref> The substrate opens and closes to allow the entry of the protein to be deubiquitinased. | ||
| - | The enzyme take this configuration thanks to <scene name='86/868189/H_bonds_around_ser177/1'>many hydrogen bonds around Ser177.</scene> This is why | + | The enzyme take this configuration thanks to <scene name='86/868189/H_bonds_around_ser177/1'>many hydrogen bonds around Ser177.</scene> This is why phosphorylation is so important to the function of the enzyme. The phosphate group forms many links between substrate ubiquitin and a segment of the OTU domain. This is rare among the known structures of deubiquitinases. Phosphorylation-driven conformational change ressembles the one of [https://en.wikipedia.org/wiki/Kinase kinases]. |
== Biological role == | == Biological role == | ||
| Line 65: | Line 65: | ||
== References == | == References == | ||
<references/> | <references/> | ||
| - | |||
| - | Ubiquitin https://fr.wikipedia.org/wiki/Ubiquitine | ||
| - | |||
| - | Ubiquitination https://fr.wikipedia.org/wiki/Ubiquitination | ||
| - | |||
| - | Cysteine protease https://fr.wikipedia.org/wiki/Prot%C3%A9ase_%C3%A0_cyst%C3%A9ine | ||
| - | |||
| - | Microtubule https://fr.wikipedia.org/wiki/Microtubule | ||
Revision as of 18:08, 23 January 2021
| This Sandbox is Reserved from 26/11/2020, through 26/11/2021 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1643 through Sandbox Reserved 1664. |
To get started:
More help: Help:Editing |
3TMP
| |||||||||||
References
- ↑ Mukhopadhyay D, Riezman H. Proteasome-independent functions of ubiquitin in endocytosis and signaling. Science. 2007 Jan 12;315(5809):201-5. doi: 10.1126/science.1127085. PMID:17218518 doi:http://dx.doi.org/10.1126/science.1127085
- ↑ Schnell JD, Hicke L. Non-traditional functions of ubiquitin and ubiquitin-binding proteins. J Biol Chem. 2003 Sep 19;278(38):35857-60. doi: 10.1074/jbc.R300018200. Epub 2003, Jul 14. PMID:12860974 doi:http://dx.doi.org/10.1074/jbc.R300018200
- ↑ Wilkinson KD. Regulation of ubiquitin-dependent processes by deubiquitinating enzymes. FASEB J. 1997 Dec;11(14):1245-56. PMID:9409543
- ↑ Amerik AY, Hochstrasser M. Mechanism and function of deubiquitinating enzymes. Biochim Biophys Acta. 2004 Nov 29;1695(1-3):189-207. doi:, 10.1016/j.bbamcr.2004.10.003. PMID:15571815 doi:http://dx.doi.org/10.1016/j.bbamcr.2004.10.003
- ↑ Urbe S, Liu H, Hayes SD, Heride C, Rigden DJ, Clague MJ. Systematic survey of deubiquitinase localization identifies USP21 as a regulator of centrosome- and microtubule-associated functions. Mol Biol Cell. 2012 Mar;23(6):1095-103. doi: 10.1091/mbc.E11-08-0668. Epub 2012, Feb 1. PMID:22298430 doi:http://dx.doi.org/10.1091/mbc.E11-08-0668
- ↑ Huang OW, Ma X, Yin J, Flinders J, Maurer T, Kayagaki N, Phung Q, Bosanac I, Arnott D, Dixit VM, Hymowitz SG, Starovasnik MA, Cochran AG. Phosphorylation-dependent activity of the deubiquitinase DUBA. Nat Struct Mol Biol. 2012 Jan 15;19(2):171-5. doi: 10.1038/nsmb.2206. PMID:22245969 doi:10.1038/nsmb.2206
- ↑ https://authors.library.caltech.edu/261/1/AMBpb04.pdf
- ↑ Das C, Hoang QQ, Kreinbring CA, Luchansky SJ, Meray RK, Ray SS, Lansbury PT, Ringe D, Petsko GA. Structural basis for conformational plasticity of the Parkinson's disease-associated ubiquitin hydrolase UCH-L1. Proc Natl Acad Sci U S A. 2006 Mar 21;103(12):4675-80. Epub 2006 Mar 13. PMID:16537382
- ↑ Amerik AY, Hochstrasser M. Mechanism and function of deubiquitinating enzymes. Biochim Biophys Acta. 2004 Nov 29;1695(1-3):189-207. doi:, 10.1016/j.bbamcr.2004.10.003. PMID:15571815 doi:http://dx.doi.org/10.1016/j.bbamcr.2004.10.003
- ↑ Singhal S, Taylor MC, Baker RT. Deubiquitylating enzymes and disease. BMC Biochem. 2008 Oct 21;9 Suppl 1:S3. doi: 10.1186/1471-2091-9-S1-S3. PMID:19007433 doi:http://dx.doi.org/10.1186/1471-2091-9-S1-S3
- ↑ Sun J, Shi X, Mamun MAA, Gao Y. The role of deubiquitinating enzymes in gastric cancer. Oncol Lett. 2020 Jan;19(1):30-44. doi: 10.3892/ol.2019.11062. Epub 2019 Nov 7. PMID:31897112 doi:http://dx.doi.org/10.3892/ol.2019.11062
- ↑ Saldana M, VanderVorst K, Berg AL, Lee H, Carraway KL. Otubain 1: a non-canonical deubiquitinase with an emerging role in cancer. Endocr Relat Cancer. 2019 Jan 1;26(1):R1-R14. doi: 10.1530/ERC-18-0264. PMID:30400005 doi:http://dx.doi.org/10.1530/ERC-18-0264