Journal:Acta Cryst D:S205979832100677X
From Proteopedia
(Difference between revisions)

| Line 11: | Line 11: | ||
*<scene name='88/886503/Cv/22'>The dimer formation of WcAG with 180ᴼ rotation</scene>. | *<scene name='88/886503/Cv/22'>The dimer formation of WcAG with 180ᴼ rotation</scene>. | ||
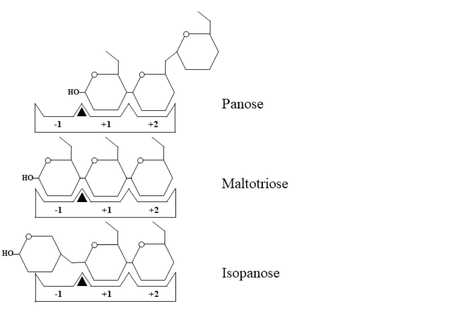
| - | ''Wc''AG formed a <scene name='88/886503/Cv/26'>homodimer, of which the N-terminal domain of one monomer orientated in proximity to the catalytic domain of another</scene>, creating the substrate-binding groove. The residues near the dimer interface are shown in dark blue and deep sky blue sticks, while the maltotriose and catalytic residues are represented by yellow and magenta, respectively. The active site of ''Wc''AG was naturally designed to fit perfectly with maltotriose | + | ''Wc''AG formed a <scene name='88/886503/Cv/26'>homodimer, of which the N-terminal domain of one monomer orientated in proximity to the catalytic domain of another</scene>, creating the substrate-binding groove. The residues near the dimer interface are shown in dark blue and deep sky blue sticks, while the maltotriose and catalytic residues are represented by yellow and magenta, respectively. The active site of ''Wc''AG was naturally designed to fit perfectly with maltotriose. |
Ligand binding sites: | Ligand binding sites: | ||
| Line 26: | Line 26: | ||
{{Clear}} | {{Clear}} | ||
<scene name='88/886503/Cv/16'>The distortion of the glucose ring in maltose-WcAG intermediate structure</scene> (grey) (PDB ID: [[7ehi]]) is clearly observed by superimposition with maltotriose in ''Wc''AG structure (cyan) (PDB ID: [[7dcg]]). | <scene name='88/886503/Cv/16'>The distortion of the glucose ring in maltose-WcAG intermediate structure</scene> (grey) (PDB ID: [[7ehi]]) is clearly observed by superimposition with maltotriose in ''Wc''AG structure (cyan) (PDB ID: [[7dcg]]). | ||
| + | |||
| + | The <scene name='88/886503/Cv2/4'>Arg-Glu salt bridge gate (R176-E296)</scene> in front of the active site modulates the substrate specificity of ''Wc''AG. This scene represents the movement of R176, E296 and F295 at two loops (P174-Y180 and T290-D300) in front of the active site when there is no substrate bound (grey) and when the active site is occupied by acarbose (salmon). | ||
<b>References</b><br> | <b>References</b><br> | ||
Revision as of 13:26, 5 July 2021
| |||||||||||
This page complements a publication in scientific journals and is one of the Proteopedia's Interactive 3D Complement pages. For aditional details please see I3DC.