We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1692
From Proteopedia
(Difference between revisions)
| Line 22: | Line 22: | ||
== Other important features == | == Other important features == | ||
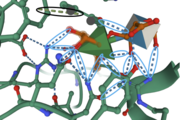
An <scene name='89/892735/Acetate_ion_interactions/1'>acetate ion</scene> is bound to the substrate complex, forming hydrogen bonds with amino acid residues Arg166 and Tyr202. The acetate ion provides more opportunity for the substrate to form interactions with amino acids near, or within, the active site, and may contribute to the elimination of the glycosidic linkages. | An <scene name='89/892735/Acetate_ion_interactions/1'>acetate ion</scene> is bound to the substrate complex, forming hydrogen bonds with amino acid residues Arg166 and Tyr202. The acetate ion provides more opportunity for the substrate to form interactions with amino acids near, or within, the active site, and may contribute to the elimination of the glycosidic linkages. | ||
| + | |||
<scene name='89/892735/Surfaceview/2'>Here</scene>, I have provided a surface view of the substrate (Rha) bound into the enzyme. This view indicates how deep the cleft of the active site is as well as other areas within the structure. This view is important for indicating how other proteins or substrates interact with this enzyme. For example, you can use this view to get an idea if a substrate is too big to fit into the active site. | <scene name='89/892735/Surfaceview/2'>Here</scene>, I have provided a surface view of the substrate (Rha) bound into the enzyme. This view indicates how deep the cleft of the active site is as well as other areas within the structure. This view is important for indicating how other proteins or substrates interact with this enzyme. For example, you can use this view to get an idea if a substrate is too big to fit into the active site. | ||
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Revision as of 23:01, 8 December 2021
| This Sandbox is Reserved from 10/01/2021 through 01/01//2022 for use in Biochemistry taught by Bonnie Hall at Grand View University, Des Moines, USA. This reservation includes Sandbox Reserved 1690 through Sandbox Reserved 1699. |
To get started:
More help: Help:Editing |
Structure and Function of FoRham1
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 Kondo T, Kichijo M, Maruta A, Nakaya M, Takenaka S, Arakawa T, Fushinobu S, Sakamoto T. Structural and functional analysis of gum arabic l-rhamnose-alpha-1,4-d-glucuronate lyase establishes a novel polysaccharide lyase family. J Biol Chem. 2021 Jul 22:101001. doi: 10.1016/j.jbc.2021.101001. PMID:34303708 doi:http://dx.doi.org/10.1016/j.jbc.2021.101001